rate and extent of chemical reactions topic 6
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
in a graph with time against quantty of product formed how can get an idea of the rate of reaction
the slope of the line gives us an idea of the rate of reaction -the steeper the slope the faster the reaction
why is the reaction fast at the beginning and what happens as time goes
because we have a large number of reactant molecules so lots of them are reacting and forming the product and reaction will start to slow down because a lot of the reactant molecules have turned into product which means there are fewer molecules to react. at the end all of the reactant molecules have already reacted
how is the quantity of reactant or product be measured
The quantity of reactant or product can be measured by the mass in grams or by a volume in cm3 .
how can the rate of a chemical reaction be found
The rate of a chemical reaction can be found by measuring the quantity of a reactant used or the quantity of product formed over time:
mean rate of reaction = quantity of reactant used/time taken
mean rate of reaction = quantity of product formed/time taken
the quantity of reactant or product can be measured
The quantity of reactant or product can be measured by the mass in grams or by a volume in cm3 .
the units of rate of reaction is
The units of rate of reaction may be given as g/s or cm3 /s.
Factors which affect the rates of chemical reactions include:
the concentrations of reactants in solution, the pressure of reacting gases, the surface area of solid reactants, the temperature and the presence of catalysts.
collision theory
chemical reactions can only take place when the reacting particles collide with each other. the collisions must have sufficient energy
the rate of a chemical reaction is determined by the frequency of successful collisions
effect of the product if a higher concentration of reactant
more product because we started with more reactant molecules
required practical 5
use a measuring cylinder to put 10cm3 of sodium thiosulfate solution into a conical flask
place the conical flask onto a printed black cross
next, add 10cm3 of hydrochloric acid into the conical flask
swir the solution and start a stopwatch
look down through the top of the flask
after a certain time, the solution will turn cloudy
we stop the clock when we can no longer see the cross
carry out the experiment again using lower concentrations of sodium thiosulfate solution
repeat the whole experiment and calculate mean values for each concentration of sodium thiosulfate soluton
when is a measurement reproducible
if it can be repeated by another person or be repeated by another person by using a different technique or equipment and still get the same result
what is a problem with the required practical
different people have different eyesights meaning that some people may see the cross for longer than others and so may not get the same results but because all the students use the same size cross the problem may not be too great
second practical
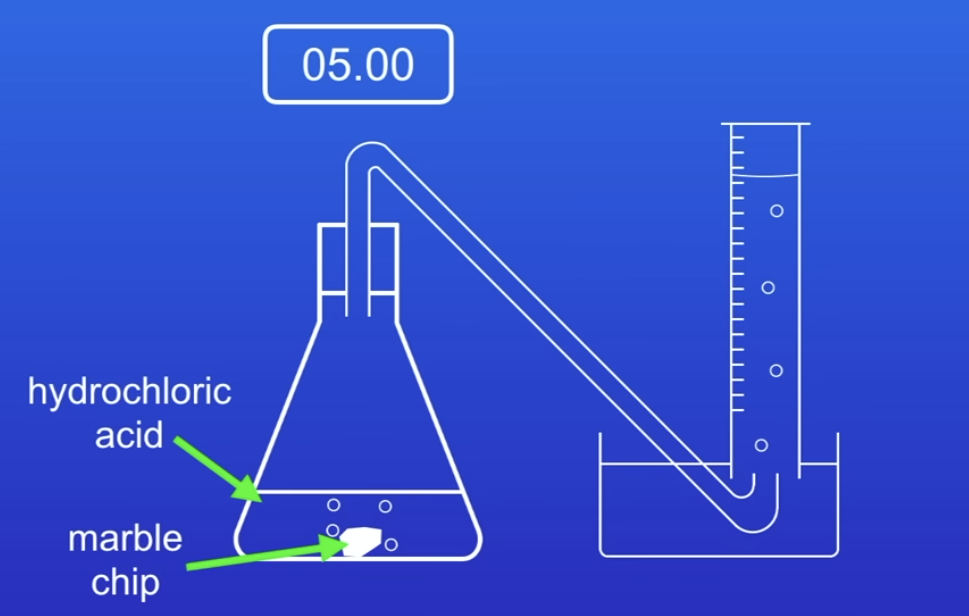
-use a measuring cylinder to place 5cm3 of hydrochloric acid into a conical flask
-attach the conical flask to. bung and delivery tube
-place the delivery tube into a container filled with water
-then place an upturned measuring cylinder also filled with water over the delivery tube
-add 3cm strip of magnesium to hydrochloric acid and start stopwatch
-the reaction produces hydrogen gas which is trapped in the measuring cylinder
-every 10secs, measure the volume of hydrogen gas in the measuring cylinder
-continue until no more hydrogen is given off
-repeat experiment using different concentrations of hydrochloric acid
Effect of Surface Area on Rate"
rate increases when surface area increases
-smaller sized blocks of solid reactants have a greater surface area to volume ratio than larger blocks. this means that they have more particles on the surface so there are more collisions per second which increases rate of reaction
one way of investigating the effect of surface area rate of reaction
-marble chips contain calcium carbonate which reacts with hydrochloric acid to produce carbon dioxide gas
-we can measure the volume of carbon dioxide gas and use this to determine the rae of reaction
-then we can change the surface area of the marble of chips
-measuring the volume of gas can be difficult using a measuring cylinder like that because bubbles can be quite rapid and so we can get more accurate results by using a gas syringe or by measuring the mass of carbon dioxde gas thats lost
another way of investigating
as carbon dioxide is produced, the mass is decreases
-we place our reaction on a balance and as carbon dioxide is produced the mass decreases and we use this to calculate the rate of reaction
the cotton wool prevents the acid from splashing out of the flask but also leets the carbon dioxide out and that would cause mass to drop more than it should causing anomalous result
actvation energy
the activation energy is the minimum amount of energy that the particles must have in order to react(collide successfully)
increasing temperature affect rate
will increase rate because increasing the temperature increases the energy of particles. Because the particles now have more energy, they now move faster and increases the frequency of collisions, there is a greater number of collisions per second
what do catalysts do to reaction
increase the rate of chemical reactions but are not used up during the reaction by providing a different pathway for the reaction that has a lower ac
key points about catalysts
-do not include catalysts in chemical equations for a reaction because not used up in the reaction and so are not a reactant
-different reactions need different catalysts
-enzymes act as catalysts in living organisms