IMED1003 - Fatty Acid Oxidation (L17)
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
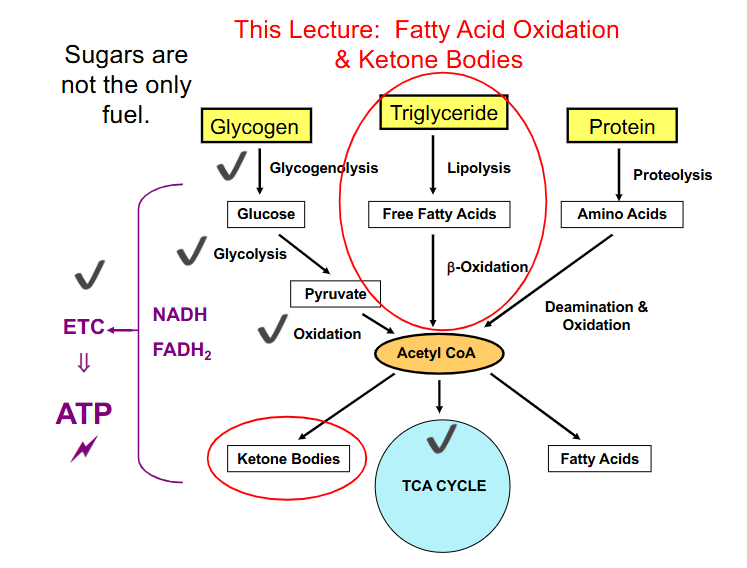
Summary so Far
DIAGRAM ON SLIDE 3
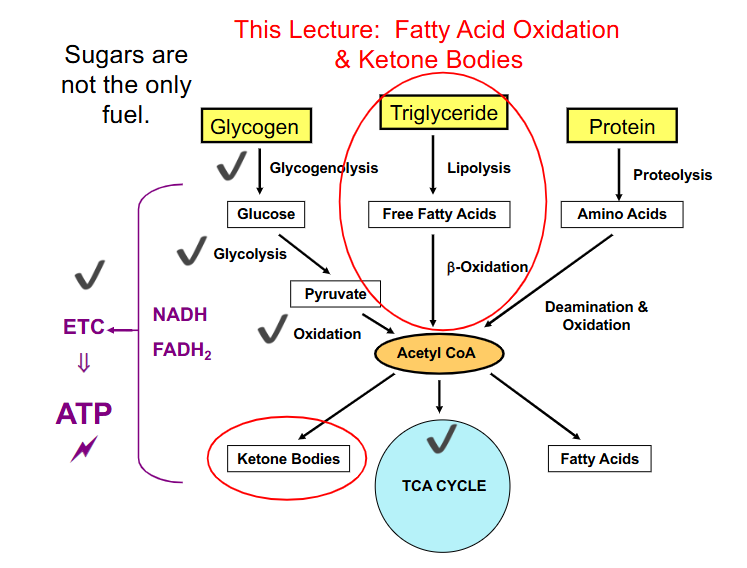
IMPORTANT THING ABOUT ACETYL COA
- once the 2 carbon acetyl CoA is formed there is no going back to form Glucose or pyruvate
- it will advance into the TCA cycle
- thats why we turn FFAs into ketone bodies from acetyl CoA
What we need to know
DIAGRAM ON SLIDE 4
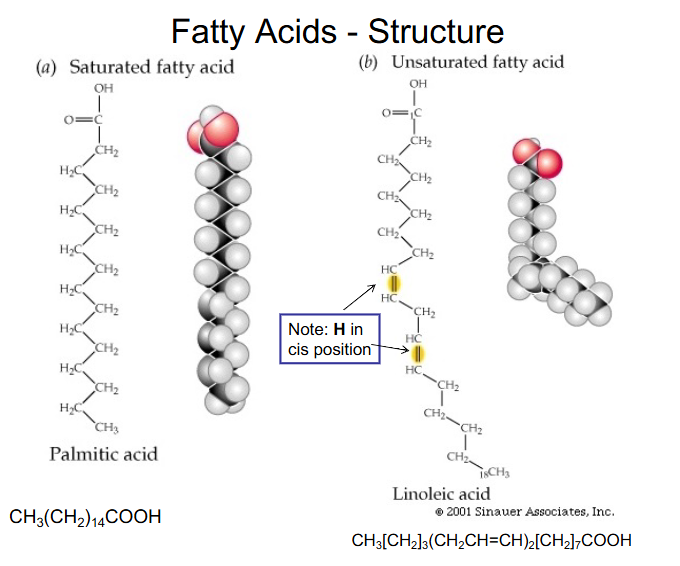
Fatty Acids - Structure
- Fatty acids = alkyl chain with terminal carboxyl group
- saturated (no C=C bonds) or unsaturated
- basic formula CH3-(CH2)n-COOH (completely saturated)
- Unsaturated - up to 6 double bonds per chain (almost always in the cis configuration)
- transported in circulation by albumin
- we call the carboxyl carbon the number 1 carbon
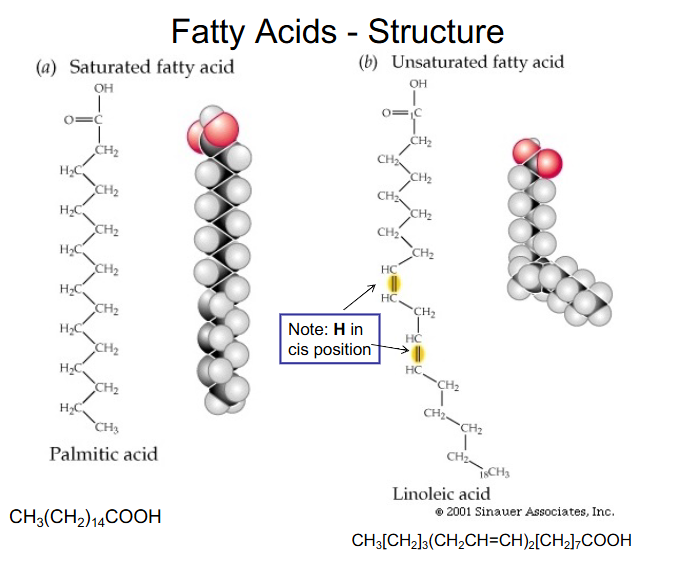
Fatty Acids - Structure DIAGRAM
DIAGRAM ON SLIDE 6
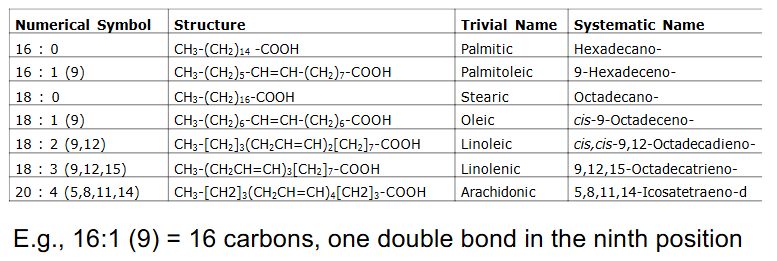
Common Fatty Acids
- Most common FA in biological systems have even number of Carbon atoms (e.g C16, C18, C20)
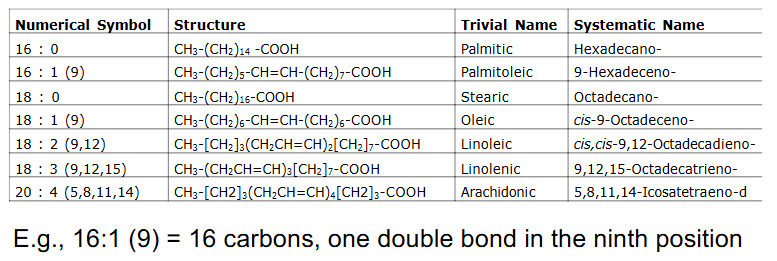
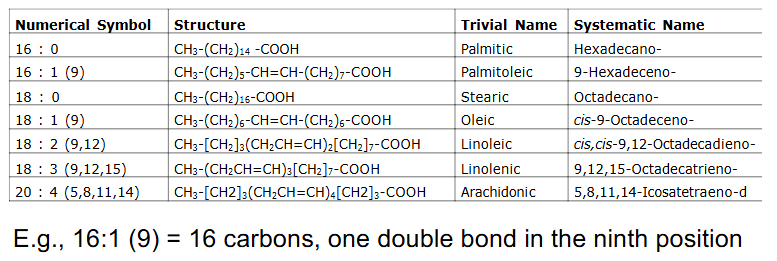
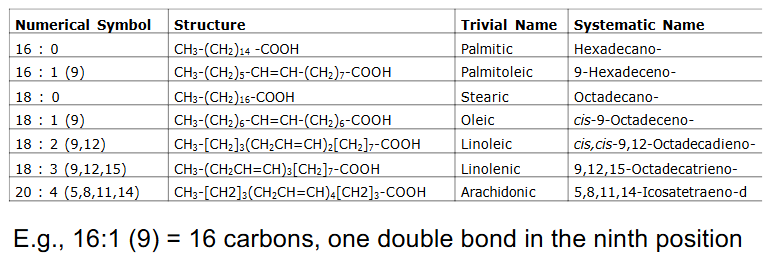
Fatty Acid Nomenclature
- e.g 16:1 (9) = 16 carbons, one double bond in 9th position
- we only consider oxidation of even number of C, saturated, long chain FA
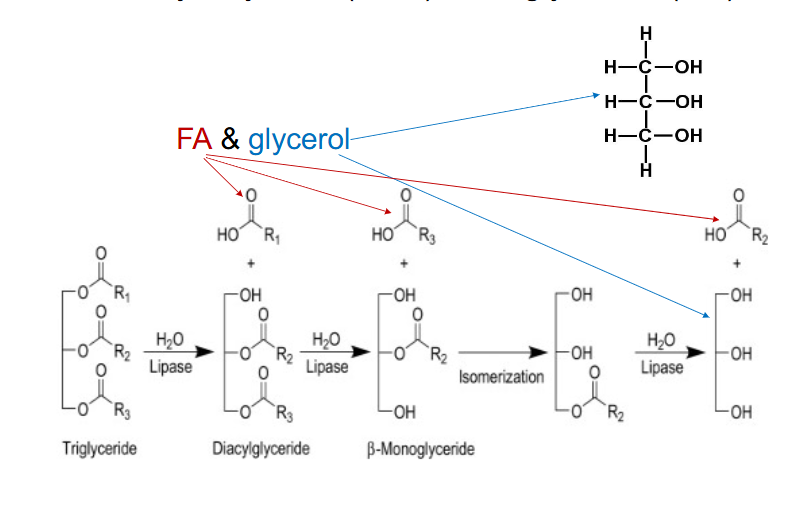
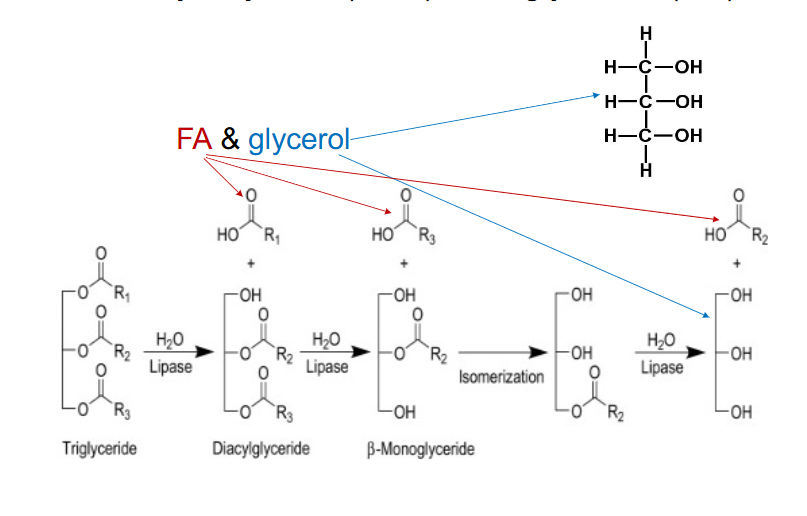
Triacyl Glycerol (TAG) or Triglyceride (TG)
- energy in TG not directly available
- Hydrolysed by lipases (e.g pancreatic lipases)
- Lipases break ester bonds
- Release FA and glycerol
Glycerol and Fatty Acids as Energy Source
- carbons within a glycerol can be converted to dihydroxy acetone phosphate (DHAP) which is an intermediate of glycolysis and therefore can contribute to glucose (glycerol is glucogenic)
- fatty acids are not glucogenic, they are ketogenic
TG efficient Energy "storage"
ON A PER WEIGHT BASIS:
- Yield is around 2+ times as much ATP as glycogen
- Store without association with H2O all around body
- Glycogen is hydrophillic binds 2x its weight in H2O. Therefore need >2 times tissue weight of glycogen for E from same weight in fat
- CH2O store lasts around 24h of fasting, largest stores in liver and skeletal muscle, within cells and often just used by that cell (skeletal muscle is greedy) - fat supply lasts several weeks (or more) and all over the body - can supply lots of different cells
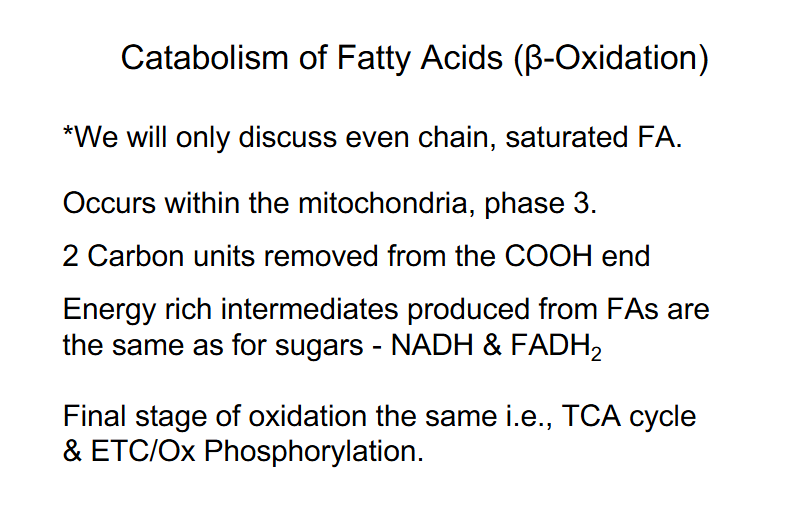
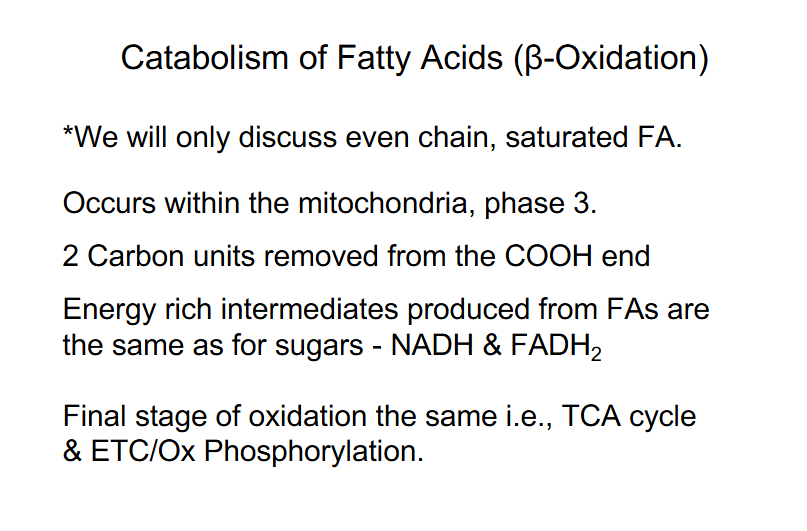
Catabolism of Fatty Acids PHASES
OCCURS IN 3 PHASES:
PHASE 1: Activation of Fatty Acid (FA) (occurs in the cytosol)
PHASE 2: Transport activated FA into mitochondria
PHASE 3: Beta oxidation - mitochondria (successive removal of 2C units -> Acetyl CoA
.
- much like glycolysis, we need to input energy to get a lot of energy from FA, which is what the activation stage is
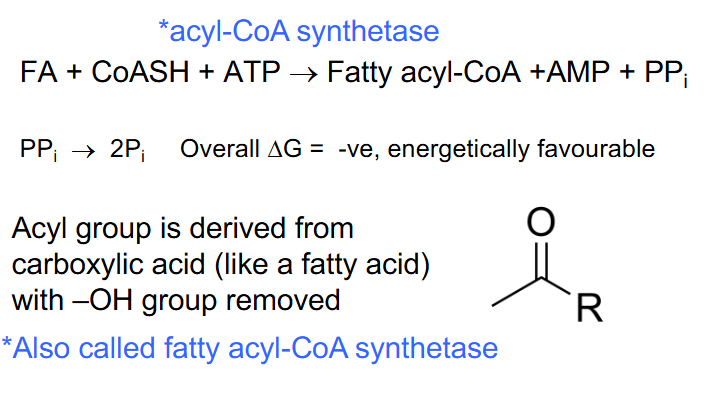
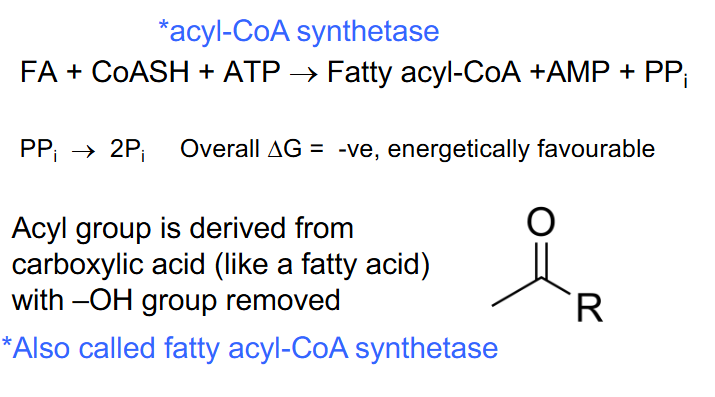
FA Catabolism - Phase 1 (FA ACTIVATION)
- in cytosol initial ATP investment *acyl-CoA synthetase
.
FA + CoASH + ATP -> Fatty acyl-CoA + AMP + PPi
.
PPi -> 2Pi (overall delta G is very negative)
.
- Acyl group is derived from carboxylic acid (like a fatty acid) with -OH group removed
*also called fatty acyl-CoA synthetase
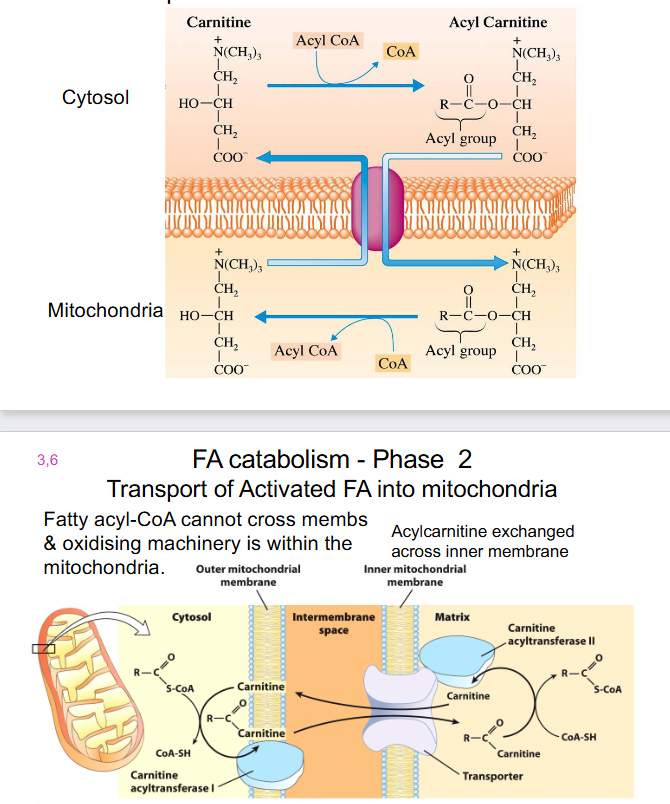
FA Catabolism - Phase 2
- Transport of Activated FA into mitochondria
- Fatty Acyl-CoA cannot cross membs and oxidising machinery is within the mitochondria
- acylcarnitine exchanged across inner membrane
- carnitine carrier: fatty acyl group now attached to carnitine
- Fatty acyl group then transferred back to CoA, removing fatty acyl-CoA
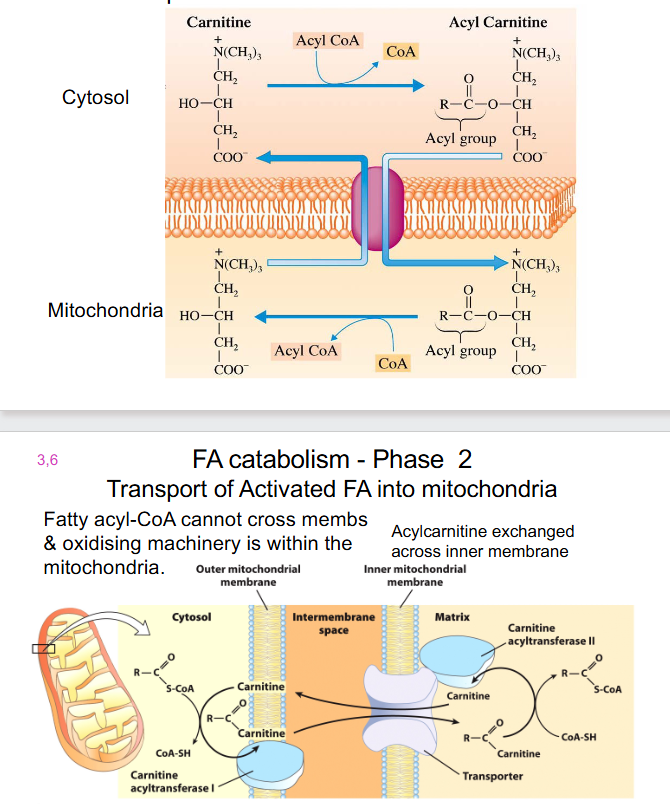
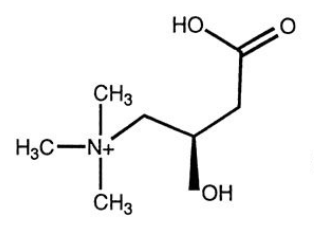
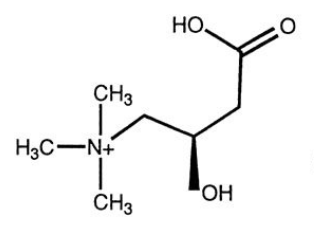
Carnitine
- long chain FA cannot diffuse into mitochondria, must be transported via a carrier:
- carnitine (can be synthesised from lysine and methionine)
- Diet: in red meats and dairy products
- People with low carnitine levels often have lipid deposition in muscles, becomes irritable and weak
- Severe disorders can be fatal.
FA Catabolism - Phase 3 - Beta-Oxidation of Fatty Acids
- spiral pathway, multi-enzyme complex, get quick processing
- 2 Carbon units removed from COOH end
- series of reactions, rearrangement of bonds
- Final cleavage releases:
- Acetyl CoA, 2C shorter FA-CoA (reenters spiral)
- Energy rich intermediates produced: NADH and FADH2 (same as glucose)
- Final stage of FA oxidation = TCA cycle and ETC
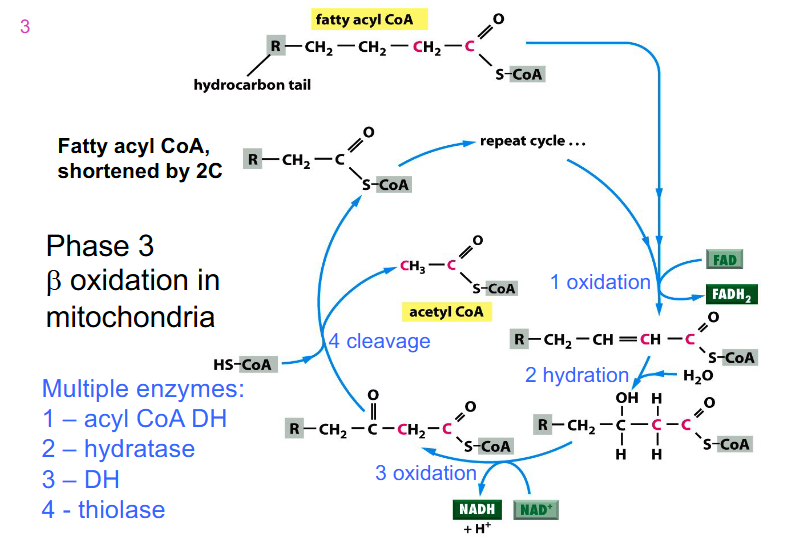
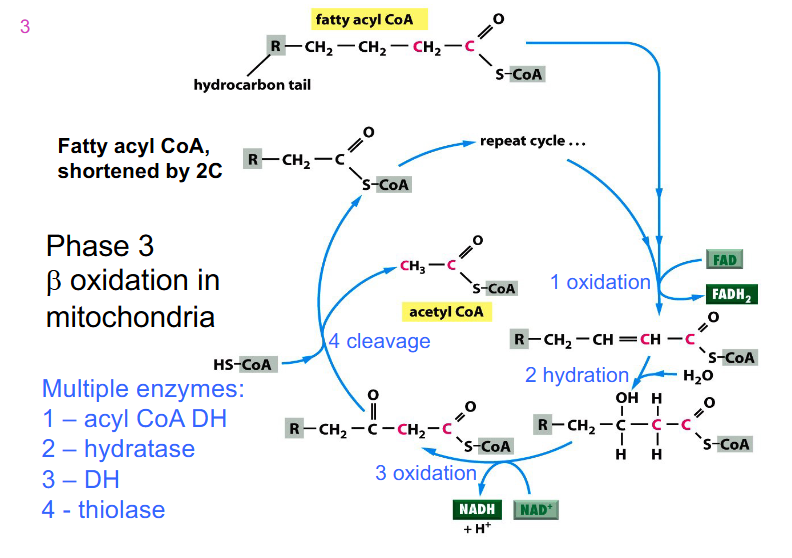
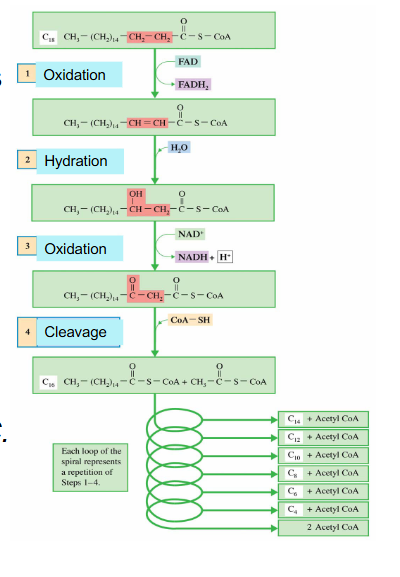
Phase 3 Cycle DIAGRAM
- we start off with fatty acyl CoA which was activated in the cytosol
- mulitple enzymes, not assessed on the enzymes
- process is repeated until we have 4 carbon fatty acyl CoA and then we have final sequence of reactions
- we feed in fatty acyl CoA and it undergoes an oxidation reaction
- most oxidation redox reactions are catalysed by dehydrogenases
- FAD is reduced to FADH2 while the carbons are oxidised (2 ATPs produced)
- another one is reducing NAD+ to NADH while carbons are oxidised (3 ATP produced)
- acetyl CoA is cleaved (step 4) and we feed in another Coenzyme A to join the other carbons that we still have on fatty acid chain
- so the final fatty Acyl CoA is 2 carbons shorter than what we started with after one cycle
- this new substance is fed back into the cycle
- as we get shorter, the last cycle has an acetyl CoA formed (it goes from 4 to 2)
Beta Oxidation
- accounts for bulk of energy production from fatty acids in humans
- Control exerted by availability of substrates and cofactors. e.g carnitine shuttle of FA into mitochondria
- Must be supplemented by other mechanisms to deal with odd chain and unsaturated FA (require additional enzymes)
- there are also minor pathways
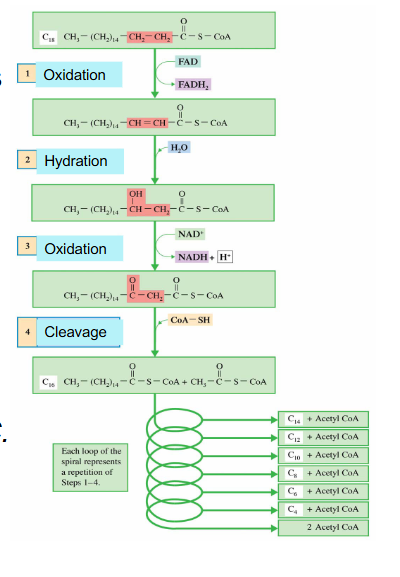
EXAMPLE of Beta Oxidation with 18 carbon stearic acid
- 18:0 stearic acid
- Spiral repeats, FA becomes shorter by 2 carbons with each turn
.
All turns (except last) give:
- 1 NADH and 1 FADH2
- 1 AcCoA for each turn and 2 AcCoA at the LAST step
.
AcCoA --> TCA Cycle, and ETC and oxidative phosphorylation
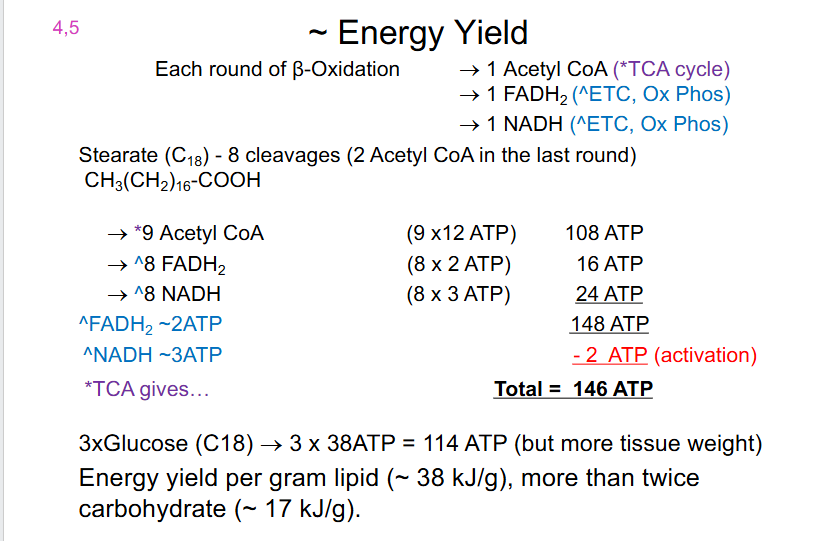
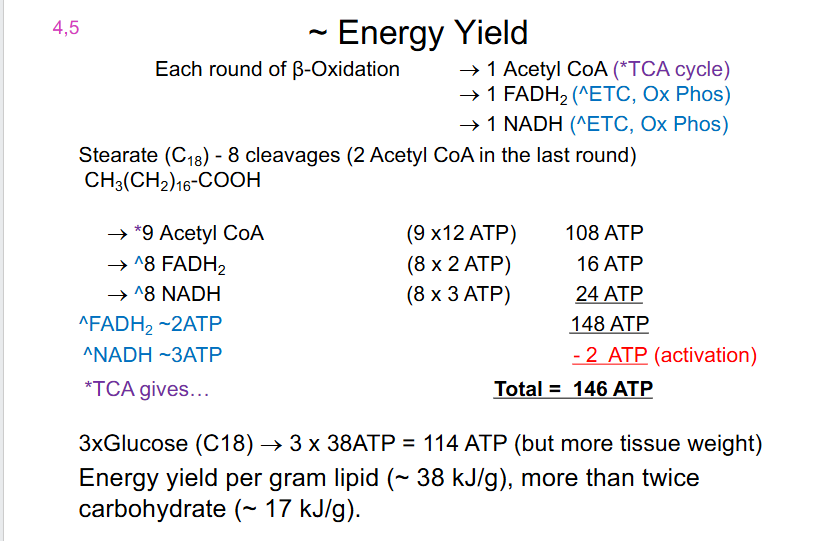
Energy Yield
EACH ROUND OF BETA OXIDATION:
- 1 Acetyl CoA (*TCA Cycle)
- 1 FADH2 (ETC, Ox Phos)
- 1 NADH (ETC, Ox Phos)
.
Stearate (C18) - 8 cleavages (2 Acetyl CoA in the last round) (CH3(CH2)16-COOH)
- 9 Acetyl CoA (9 x 12 = 108 ATP)
- ^8 FADH2 (8 x 2 = 16 ATP)
- ^8 NADH (8 x 3 = 24 ATP)
- Total is 148 ATP but 2 ATP must be subtracted since that was for activation
- hence total net is 146 ATP
.
3X GLUCOSE (C18) = 3 x 38 ATP = 114 ATP (but more tissue weight)
.
- Energy yield per gram lipid (around 38kJ/g), more then twice carbohydrate (17kJ/g)
the TCA Cycle itself produces 3 NADH, 1 GTP and 1 FADH2
Preferred Fuel
- skeletal muscle at rest use fatty acids
- Active skeletal muscles use glucose (ATP provided quickly but not sustainable)
- Skeletal muscles can use ketone bodies
.
Cardiac muscle: prefers fatty acids., then ketone bodies, glucose and lactate
.
Liver prefers fatty acids
.
Brain only uses glucose and ketone bodies
.
- the reason a lot of these have specific requirements is due to enzymes present. brain does not have beta oxidation enzymes so it uses the other ones available
Clinical Perspective
Genetic deficiencies in Carnitine Transport
- mild recurrent muscle cramping - severe weakness - death
.
Primary:
- low carnitine in muscle, kidney and heart (not liver)
- Compromises long chain FA oxidation
- Overcome by dietary carnitine therapy
.
Secondary:
- genetic defect in beta-oxidation -> increased acylcarnitine
- Excreted in urine -> low levels of carnitine
- Type 1: muscle weakness, myoglobinuria
- Type 2: (infants) precipitated by fasting -> hypoketotic, cardiac malfunction and death
- treat by avoiding starvation and diet low in long chain FA
Ketone Bodies
- lipid based energy source
- water soluble (easily travels in blood to brain)
- main 2 ketone bodies are: beta-hydroxybutyrate and acetoacetate
- Main site of formation = Liver (mitochondrial matrix)
- Used by skeletal and cardiac muscle (conserve glucose for CNS)
- Used by CNS in starvation as can't use FA (don't have all beta-oxidation enzymes), so gradually switch from glucose to ketone bodies
- Neonates - precursors for cerebral lipid synthesis
.
- they are derived from acetyl CoA, liver can synthesise ketone bodies and most other tissues can use it as energy source. Liver cannot utilise them
Energy source balance (ketone bodies included)
- Usually lipid and carb metabolism balanced
- FA Oxidation -> AcCoA -> TCA Cycle + OAA -> citrate
- Pyruvate --> OAA via pyruvate carboxylase
.
What upsets the lipid/carbohydrate balance?
- Diet: high fat/low carbohydrates
- Diabetes: body cannot process glucose properly
.
- Long-term fasting: starvation
- Inadequate amount of OAA formed
- OAA present -> gluconeogenesis -> glucose
- AcCoA cannot be processed in TCA cycle
- Excess AcCoA -> ketone bodies
.
- in order for TCA Cycle to happen we need Oxaloacetate (we can also get OAA through pyruvate carboxylase)
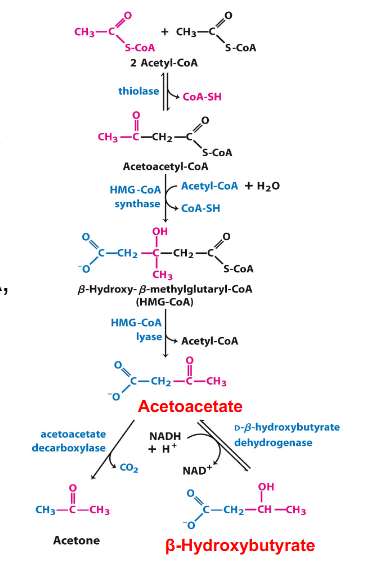
Ketone Bodies Formation in Liver
- NB: ketone bodies are acidic
- ketone bodies can be converted back into Acetyl CoA, yields 2 per ketone body
- Acetone = metabolic dead-end product, gives fruity breath
- ketone bodies is a bad name
.
- ketone bodies are produced from 2 Acetyl CoAs
- this happens in the mitochondria of the liver
- after a series of reactions, we end up with 2 acetyl groups joined together (acetoacetate) which is one of our ketone bodies
- this can be converted to beta-hydroxybutyrate, which is the other one
DONT NEED TO NOW THE REACTIONS IN DETAIL
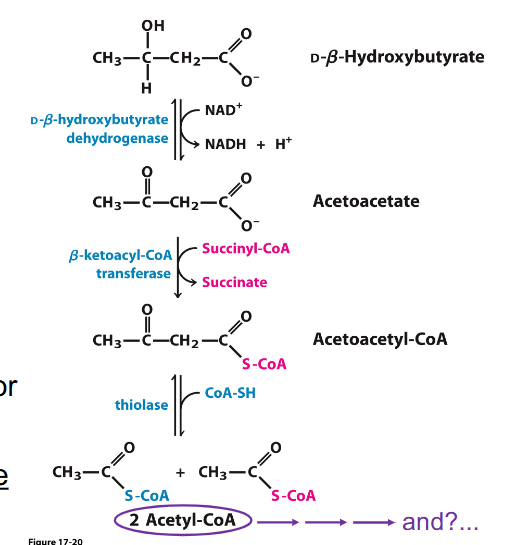
Ketone Body Reconversion
- Ketone bodies reconverted to 2 Acetyl CoA
- Acetyl CoA enters TCA cycle
- provides alternate fuel source, e.g for CNS in starvation
- Liver cannot use ketone bodies (it does not express these dehydrogenases and transferase enzymes)
Signficance of Ketone Body Formation
- overall accumulation in urine and blood = ketosis
.
Elevated ketone body formation:
- 50-100 times higher
- >20mg/100mL in blood = ketoanaemia
- >70mg/100mL flushes out of kidneys and excreted into the urine = ketonuria
.
- sweet smell of acetone is on the breath
- ketone bodies are acids
- blood can become acidifed = keto acidosis
- Diabetic ketoacidosis