🌸organic chemistry -> reactions
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
addition reaction
-> definition
-> one which two subtances react to form a single substance
addition reaction
-> notes general
-> always occur on double/triple bond
-> geometry changes from planar (unsatured C-C bond) to tetrahedral (satured C=C bond)
-> benzene doesnt undergo addition reactions due to delocalised electrons cauing stable and unreactive structure
addition reactions
-> uses 1. hydrogenation of vegetable oils
=> so this forms margarine as..
-> H2 is added with nickel (Ni) catalyst to unsaturated oils to make them partially saturated
=> also called REDUCTION reaction as we are adding hydrogen
addition reactions
-> uses 2. polymerisation reactions
-> make long chains of carbons
=> CALLED PLASTICS!!
ethene -> important organic chemical
-> undergoes many addition reactions to form organic compounds (IN NOTES)
1) ethane
2) 1,2-dichloroethane
3) chloroethane
4) ethanol
5) 1,2-dibromoethane => bromination of ethene, IMPORTANT, ionic addition
mechanism definition
-> detailed step-by-step description of how the reaction occurs.
step 1: polarisation
-> The C=C in ethene has a high concentration of negative charge.
= Br₂ is normally non-polar, but as it gets close to ethene, the electrons in the double bond repel the electrons in Br₂, polarising it.
step 2: heteroyltic fission
-> The polarisation eventually becomes so great, the molecule splits into Br- and Br+ .
-> We call this heterolytic fission because the Br- took all 2 electrons from the Br-Br bond.
step 3: carbonium ion formation
-> The Br+ attacks the electron rich C=C double bond.
-> This forms a carbonium ion
step 4: ionic attack
-> The Br- ion attacks the carbonium ion, forming 1,2-dibromoethane.
evidence for this mechanism
-> If this reaction is carried out in bromine water with some Cl- ions added, we see the normal 1,2-dibromoethane product being formed,
=> but 2 others form also; 1-bromo-2-chloroethane and 2-bromoethanol.
=> form because of the Cl- or the OH- (from water's self-ionisation) can replace the Br- in step 4.
polymerisation -> type?
addition
polymers definiton
-> long chain molecules made by joining together many small molecules (monomers)
-> they are made up of repeating units
elimination reaction definiton
-> small molecule is removed from a larger molecule
=> leaving a double bond on the larger molecule
elimination reaction notes
-> geometry changes from tetrahedral to planar
(lose a group, flatten out)
-> generally involves loss of H2O = dehydration reactions
draw the example of producing ethene gas
on paper
what happens during producing ethene gas?
-> water is removed from ethanol
what is required to remove water during a dehydration reaction?
-> Al₂O₃ (aluminium oxide) and heat
substitution reaction
-> an atom/group of atoms in a molecule
-> is replaced by another atom/group of atoms
what are the 2 sub reactions we study?
1. halogenation of alkanes
2. esterification
halogenation of alkanes -> what is it?
➞ substituting a hydrogen in an alkane with a halogen
(F, Cl, Br, I etc)
what are fully halogenated alkanes/ what do they do and why are they less common now?
➞ eg CCl4 -> all H has been replaced
➞ used as flame retardants
➞ less common as they damage the ozone layer
what is the monochlorination of methane/ethane?
➞ free-radical substitution
what is the 4 steps of halogenation of an alkane?
1. homolytic fission
2. propogation
3. propogation
4. termination
step 1. homolytic fission
➞ UV light BREAKS Cl-Cl single bond!!!!
➞ each Cl receives one of 2 electrons from the bond
➞ 2 Cl radicals now which are very reactive
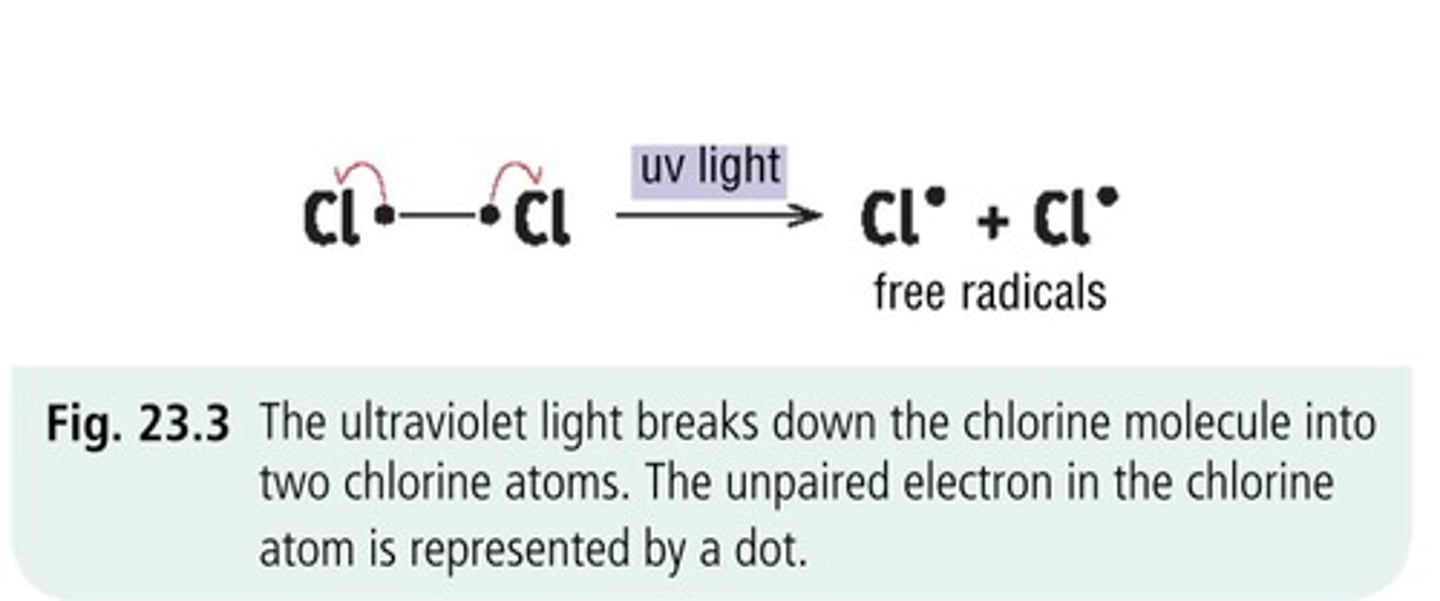
step 2. propogation
➞ ONE of the Cl free radicals ATTACKS methane molecule!! :(
➞ this forms HCl and methyl free radical
step 3. propogation
➞ this methyl radical now ATTACKS a new Cl2 molecule
➞ forms molecule of chloromethane and Cl radical
➞ Cl radical left over undergoes step 2 again
➞ chain reaction between step 2/3
step 4. termination
➞ when most of reactants have been used up during stage 2/3
➞ only small number of Cl and methyl radicals remain
➞ these combine to form Cl2, chloromethane and ethane
step 4. termination draw products
on paper
evidence for monochlorination of methane mechanism
➞ name all evidence
1. reaction takes place when exposed to UV light
2. for every photon of UV light, thousands of chloromethane molecules are formed
3. ethane is found in the products (butane)
4. adding tetramethyllead (tetraethyllead), which is source of free radicals, increases rate of reaction
5. addition of inhibitor, like O2, slows down reaction
evidence for monochlorination of methane mechanism
➞ 1. reaction takes place when exposed to UV light
➞ suggests free-radical mechanism
➞ Cl2 is broken into Cl radicals
evidence for monochlorination of methane mechanism
➞ 2. for every photon of UV light, thousands of chloromethane molecules are formed
➞ suggests chain reaction
evidence for monochlorination of methane mechanism
➞ 3. ethane is found in the products (butane)
➞ methyl(ethyl) radicals must've been present
➞ as ethane(butane) forms when 2 methyl(ethyl) radicals combine
evidence for monochlorination of methane mechanism
➞ 4. adding tetramethyllead (tetraethyllead), which is source of free radicals, increases rate of reaction
➞ only a reaction with free radicals
➞ can be sped up by using free radicals
evidence for monochlorination of methane mechanism
➞ 5. addition of inhibitor, like O2, slows down reaction
➞ shows chain reaction is taking place
➞ because O2 combines with free radicals, STOPPING chain reaction
reaction 2
-> esterification definition and process
➞ carboxylic acid reacted with alcohol
➞ H2SO4 as catalyst
➞ = ester is formed.
➞ is a SUBSTITUTION REACTION as the H on the -OH of the carboxylic acid has been substituted with an alkyl (methyl-, ethyl-, etc.)
esterification what do we need to do/what else is it?
➞ equilibrium reaction.
➞ TO DO we add WATER, called hydrolysis.
how else is hydrolysis carried out and what does this form?
➞ Hydrolysis is also carried out using a base e.g. NaOH.
➞ results in the formation of the sodium salt of the carboxylic acid.
➞ THESE salts are SOAPS.
➞ This process of hydrolysis is called saponification, in SOAP experiment
how does soap work?
➞ Soap molecules contain long, non-polar carbon chain which will dissolve oils (e.g. from skin).
➞ opposite end of the molecule contains a polar COO- Na+ which will dissolve SALTS from sweat and will also DISSOLVE in water.
➞ soap picks up oils/salts and then washes down the drain.
redox reactions
➞ in terms of O
➞ oxidation: addition of O
➞ reduction: removal of O
redox reactions
➞ in terms of H
➞ oxidation: removal of H
➞ reduction: addition of H
what is oxidation carried out using?
➞ Acidified Sodium Dichromate (Na₂Cr₂O₇ )
➞ Cr(VI) [orange] gets reduced to Cr(III) [green]
➞ Acidified Potassium Permanganate (KMnO4 )
➞ Mn(VII) [purple] gets reduced to Mn(II) [colourless].
what is reduction carried out using?
➞ Hydrogen gas and a Nickel catalyst (H₂/Ni)
what do primary alcohols get oxidised to?
➞ (ALL CHANGES HAPPEN AT END CARBON)
➞ aldehydes.
➞ excess oxidising agent
➞ = the aldehydes can then be further oxidised to carboxylic acids.
what do carboxylic acids get reduced to?
➞ aldehydes.
➞ excess reducing agent
➞ = the aldehydes can be further reduced to alcohols
what do secondary alcohols get oxidised to?
➞ ketones.
➞ cannot be oxidised further.
➞ bc carbon with oxygen is not at end of molecule.
➞ form a ketone RATHER than aldehyde.
➞ As carboxylic acids can only have the COOH on the terminal carbon, a ketone can never be oxidised to a carboxylic acid.
what do ketones get reduced to?
➞ secondary alcohols
everyday redox reaction
➞ wine left open
➞ A bottle of wine left open in air will taste "vinegary" after time.
➞ oxygen in the air oxidises the ethanol in the wine to ethanoic acid.
everyday redox reaction
➞ ethanol drinking (hangover)
➞ When ethanol is drank, the liver works hard to break it down, FOREIGN TOXIN
➞ The liver oxidises the ethanol to ETHANAL, even more toxic! ➞ HANGOVER!!
➞ Eventually ethanal is oxidised by liver to carbon dioxide and water, which are excreted through lungs and urine.
everyday redox reaction
➞ breathalyser
➞ potassium dichromate crystals [Cr(VI) - orange]
➞ drunk person breathed through tube with the crystals, the ethanol in breath would be oxidised.
➞ would reduce Cr(VI) [orange] to Cr(III) [green],
➞ ethanol was present.
alcohols reacting with sodium
➞ The H on the –OH of an alcohol acts as an acid
➞ when reacted with extremely reactive metal, sodium.
➞ forms the sodium salt of the alcohol, and hydrogen gas.
➞ DRAW IT
state 2 reasons why
H on the -COOH group is acidic?
1. inductive effect
2. stability of the carboxylate ion
why is the H on the -COOH group acidic?
1. inductive effect
➞ the O in the C=O is very electronegative
➞ C is left with a slight positive charge.
➞ draws electron density towards the C and away from the –OH, allowing H to dissociate.
why is the H on the -COOH group acidic?
2. stability of the carboxylate ion
➞ When H of the –OH dissociates to form a H+ we are left with a carboxylate ion –COO- .
➞ negative charge is not localised to just one of the oxygens but is delocalised between the two.
➞ delocalised structure gives the carboxylate ion extra stability, allowing the H+ easily dissociate.
common reactions with carboxyic acids
Acid + Base →
Acid + Base → Salt + Water
Example: CH₃COOH + NaOH → CH₃COONa + H₂O
common reactions with carboxylic acids
Acid + Carbonate →
Acid + Carbonate → Salt + Carbon Dioxide + Water
Example: 2CH₃COOH + Na₂CO₃ → 2CH₃COONa + CO₂ + H₂O
common reactions with carboxylic acids
Acid + Metal →
Acid + Metal → Salt + Hydrogen
Example: 2CH₃COOH + Mg → (CH₃COO)₂Mg + H₂
synthesis of pvc from ethene
step 1
➞ Ethene and chlorine react to form 1,2-dichloroethane.
➞ DRAW IT
synthesis of pvc from ethene
step 2
➞ Heat is used to thermally crack the 1,2-dichloroethane into chloroethene & HCl.
➞ DRAW IT
synthesis of pvc from ethene
step 3
➞ The chloroethene undergoes a polymerisation reaction to form polychloroethene (PVC)
➞ DRAW IT