Chem 28/10/25 Acidity of solutions
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Last updated 3:09 AM on 10/28/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
Ion product of water
Represented as Kw
Ion product of water is the product of the concentrations of hydrogen ions H+ and hydroxide ions OH− in water, expressed as:
As the concentration of H+ increases the PH decreases
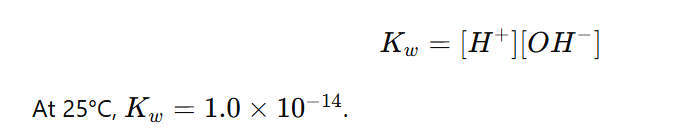
2
New cards
Temperature effect on the ion product of water
As the temperature increases the larger extent of ion dissociation
Thus more ions are formed therefore decreasing the pH (More acidity)
However the solution is still neutral
3
New cards
pH scale
a measure of how acidic or basic a substance is, ranging from
0 to 14pH = -log[H3O+']
4
New cards
The pOH
Calculated the same way you calculate pH, except its a measure of Hydroxide (OH-) concentration
pH + pOH = 14
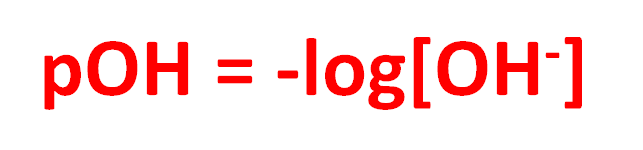
5
New cards
6
New cards
7
New cards