(Part 3) Mixtures + Separation Techniques
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What are the two kinds of mixtures and explain the difference between them
Homogenous → all the parts of the mixture are in the same state
Heterogenous → the parts of the mixture are in different states
Name these parts of a mixture
A solution
Soluble
Insoluble
Solute
Solvent
Aqueous solution
Suspension
A solution: a mixture of a solid dissolved in a liquid
Soluble: a solid that can dissolve in water
Insoluble: a solid that cannot dissolve in water
Solute: the solid that dissolves
Solvent: the fluid in which the solute dissolves in
Aqueous solution: a solution in which the solvent is water
Suspension: a mixture of fine insoluble solid particles suspended in a liquid
Explain how the particle size of an insoluble solid affects how it settles in a mixture
Large particles → settle fast → therefore don’t spend much time as a suspension
Small particles → take longer to settle → spend a long time as a suspension
What happens once the insoluble particles in a suspension settle to the bottom
It is no longer a suspension
Explain the steps to obtain Salt from Seawater
The salt pans are filled with seawater
The sun heats the rock, causing evaporation - water turns into a vapour and leaves the pan
As the water evaporated, salt crystals form by a process called crystallization
Because this process takes days, the salt crystals grow large because they are allowed to form properly
What can filtration be used for
To separate an insoluble solid from a liquid or aqueous solution
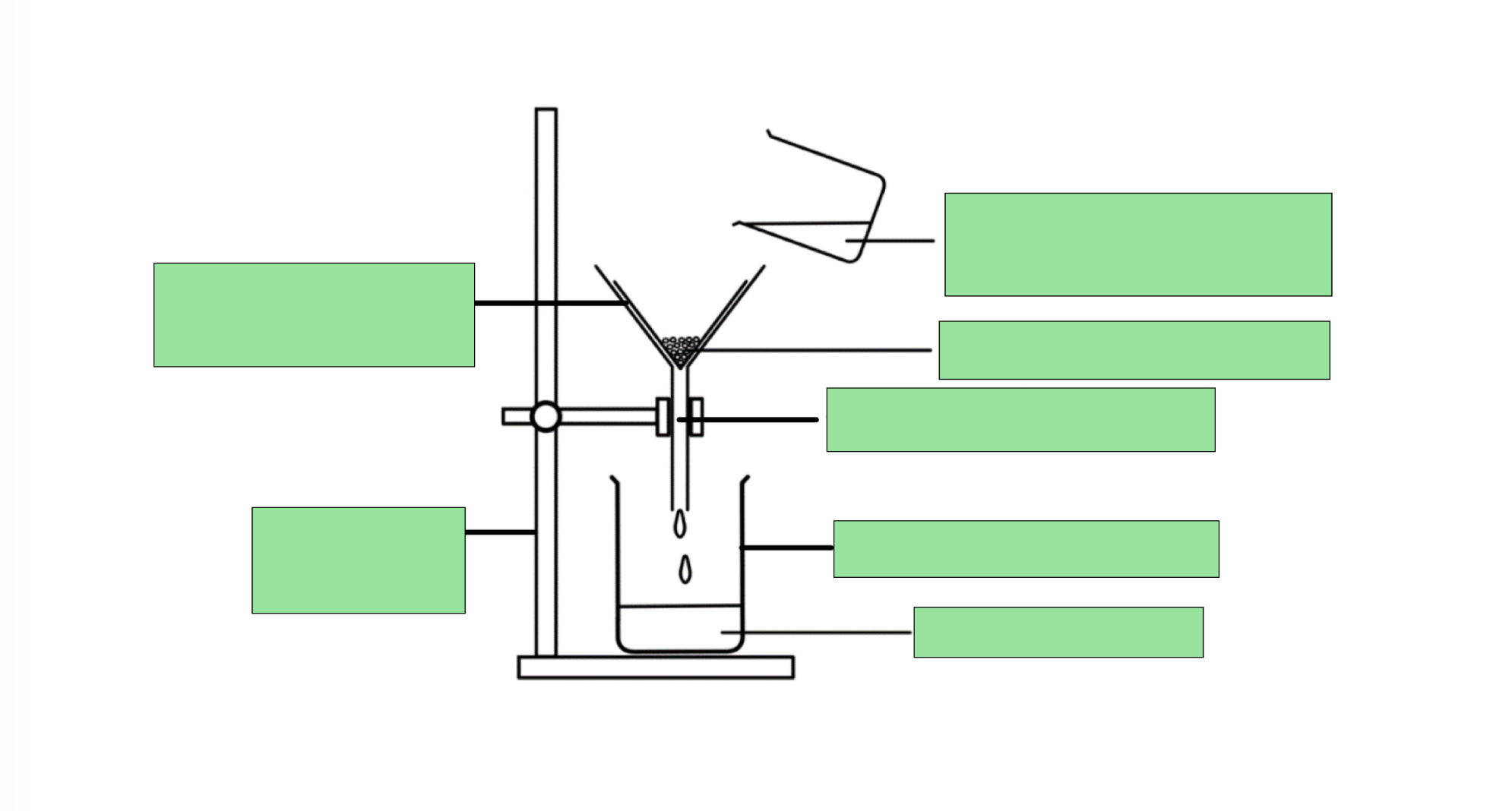
LABEL THE FILTRATION DIAGRAM
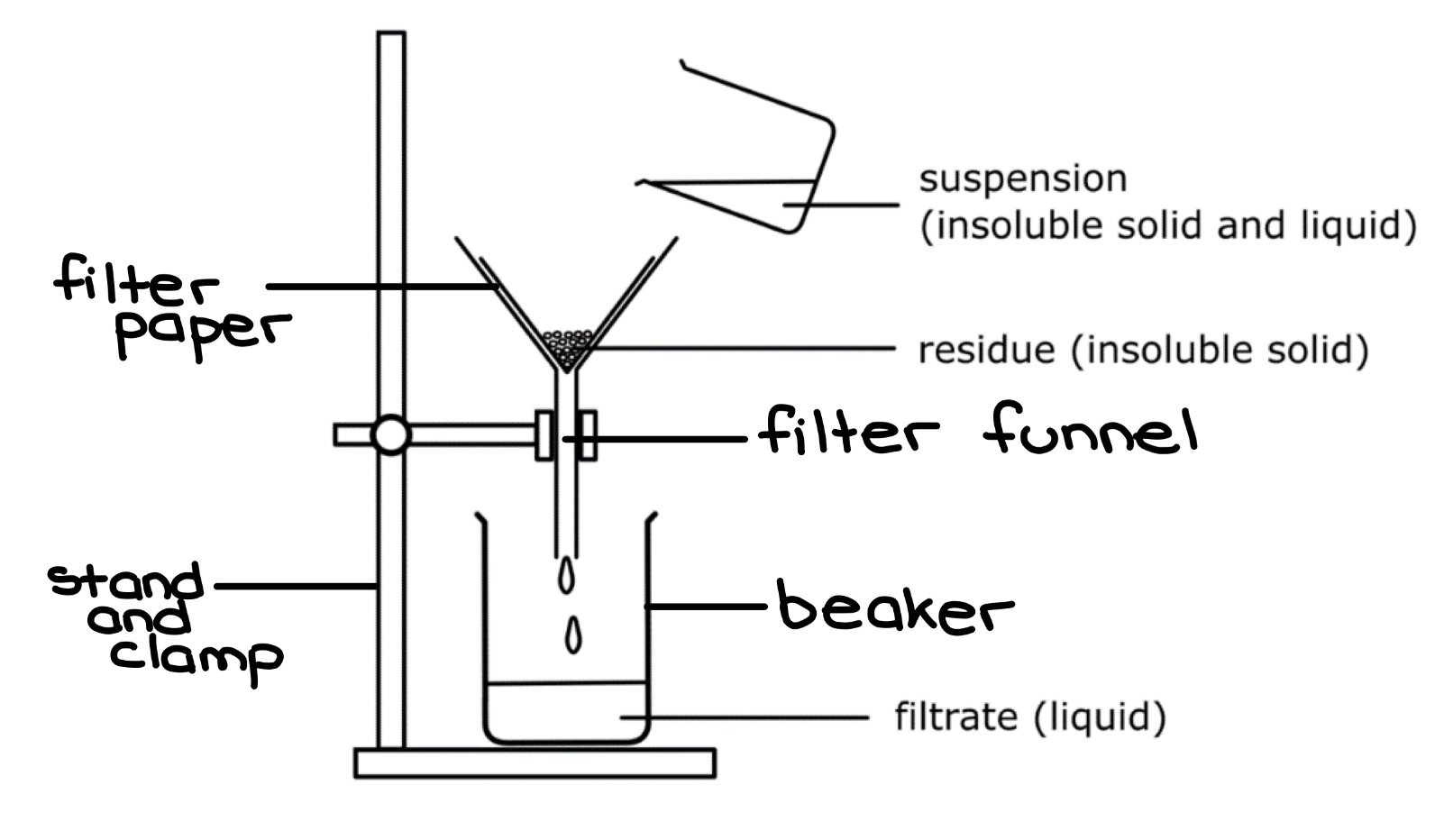
What do the terms ‘residue’ and ‘filtrate’ refer to
residue: The insoluble solid that remains on the filter paper
filtrate: The liquid or aqueous solution that passes through the filter paper
Explain why these precautions were taken while conducting filtration
A few drops of distilled water are added to the funnel before inserting the filter paper
The mixture is poured slowly during filtration
The residue is washed with distilled water
The filter paper was wet with distilled water before filtration
A few drops of distilled water are added to the funnel before inserting the filter paper → helps filter paper stay in place
The mixture is poured slowly during filtration → Prevents spilling, ensures good filtration
The residue is washed with distilled water → removes any soluble impurities/filtrate stuck behind to ensure purity
The filter paper was wet with distilled water before filtration → avoids paper absorbing product, therefore allowing a higher yield to be collected
What is evaporation to dryness used for
to obtain a soluble solid from a liquid solvent
Explain what happens in this separation technique
In this separation technique a solution (e.g. salt + water) is poured into an evaporating dish and placed over a Bunsen burner until all the solvent (water) from the solution has evaporated and only small* salt crystals are left
Why are the crystals that are left behind small
because the rate of evaporation in this case was very fast. The slower the rate of evaporation, the larger the crystals formed because they would have more time to form properly
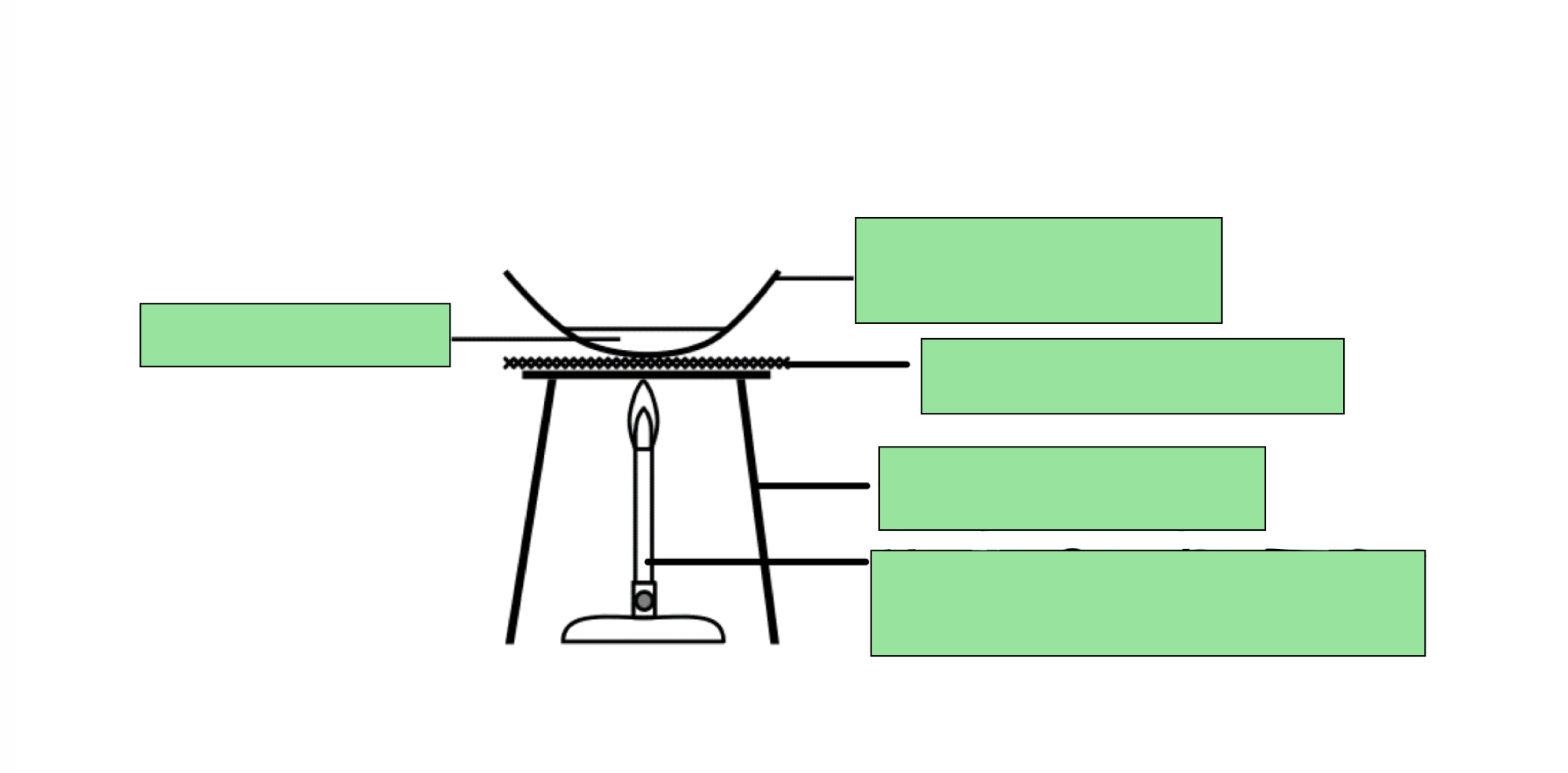
LABEL THE DIAGRAM FOR HEATING TO DRYNESS
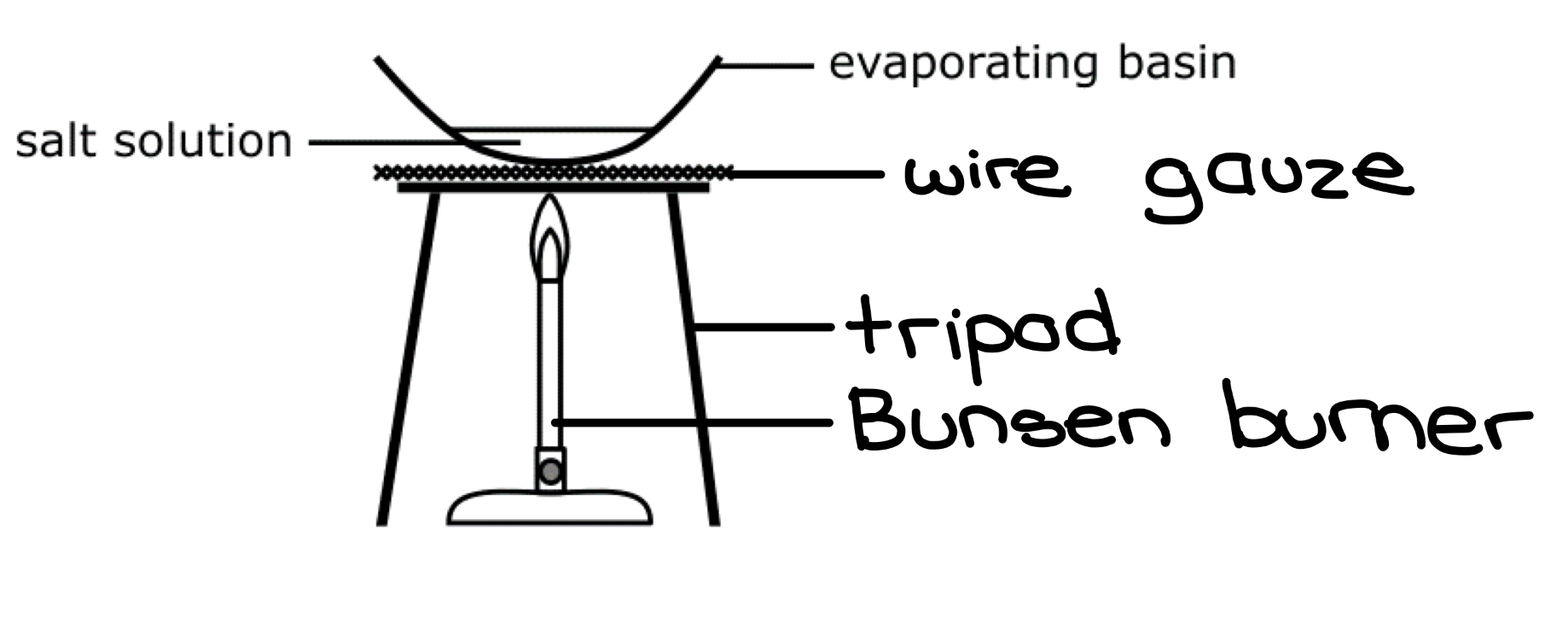
Since this technique involves direct heating from a Bunsen burner, what should this method only be used for
should only be used for salts (compounds) that are thermally stable and therefore do not decompose on heating
What would happen if heating to dryness was used on a thermally unstable salt
it might break apart or change chemically before all the water evaporates – you won’t obtain the same salt dissolved in the water
What is crystallisation used for
To obtain a soluble solid from a liquid solvent
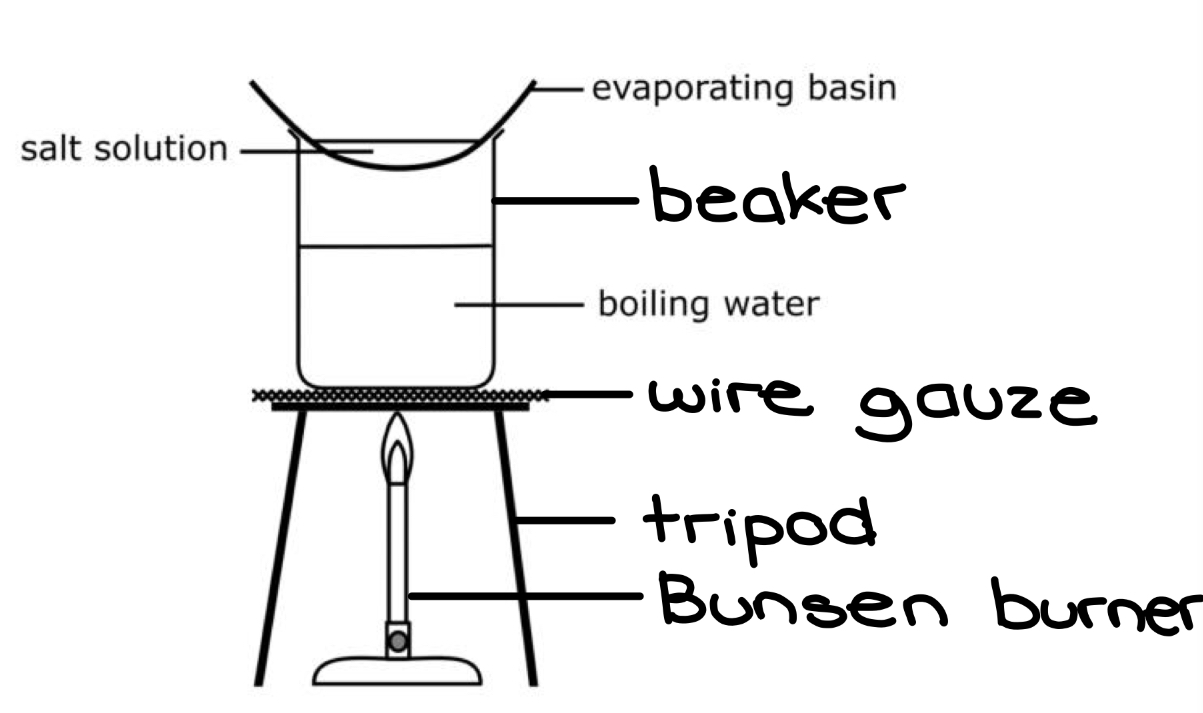
Explain how crystallisation works
First, a saturated solution (has as much solute as possible) is heated gently using a water bath until some of the water has evaporated.
The remaining solution is then allowed to cool. Since the temperature of the water is colder, it becomes less soluble (can withstand less salt dissolved in it).
Therefore, the extra solute (that the water cannot dissolve anymore) comes out of the solution and forms crystals within the solution
The remaining solution (the water + salt that can be dissolved) is super saturated
What is the difference between crystallisation and evaporation to dryness
They both achieve the same effect - but in crystallisation the crystals obtained are larger since they have more time to form properly
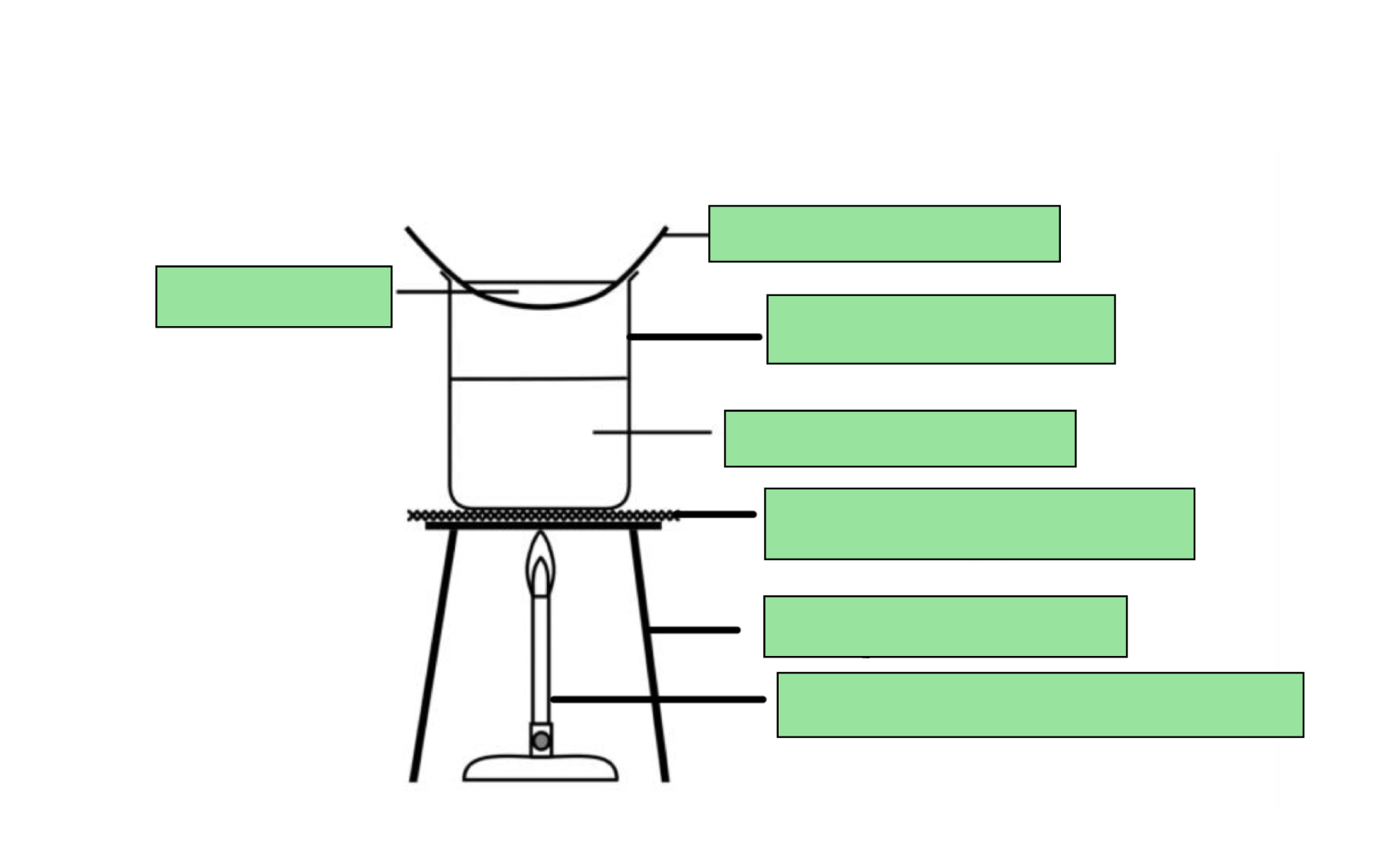
LABEL THE CRYSTALLISATION DIAGRAM
Name the three cases where crystallisation needs to be used instead of evaporation to dryness
A hydrated salt is required
The salt required is thermally unstable
Large crystals are required
Explain why if a hydrated salt is required crystallisation needs to be used
If you used evaporation to dryness on a hydrated salt, it would drive away all the water of crystallisation leaving you with the anhydrous version of that salt
Explain why if the salt required is thermally unstable crystallisation needs to be used
if a thermally unstable salt is exposed to the direct heating of the Bunsen burner in evaporation to dryness it would decompose (you wouldn’t get the same salt back)
Explain why if large crystals are required crystallisation needs to be used
Crystallisation involves evaporating water at a slow controlled rate therefore large crystals are formed, while in evaporation to dryness, the heating process is quick, so small crystals are formed