Shapes of molecules
1/25
Earn XP
Description and Tags
different names and shapes for each number of electrons with different numbers of lone pairs and bonding pairs
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
linear
total number of electron pairs: 2
total number of bonding pairs: 2
total number of lone pairs:0
bond angle: 180
total number of electron pairs: 2
total number of bonding pairs: 2
total number of lone pairs:0
bond angle: 180
linear
trigonal planar
total number of electron pairs: 3
total number of bonding pairs: 3
total number of lone pairs:0
bond angle: 120
total number of electron pairs: 3
total number of bonding pairs: 3
total number of lone pairs:0
bond angle: 120
trigonal planar
bent (v-shape)
total number of electron pairs: 3
total number of bonding pairs: 2
total number of lone pairs:1
bond angle: 118
total number of electron pairs: 4
total number of bonding pairs: 4
total number of lone pairs:0
bond angle: 109.5
tetrahedral
total number of electron pairs: 4
total number of bonding pairs: 3
total number of lone pairs:1
bond angle: 107
trigonal pyramidal
total number of electron pairs: 4
total number of bonding pairs: 2
total number of lone pairs:2
bond angle: 104.5
bent (v-shape) (2)
total number of electron pairs: 5
total number of bonding pairs: 5
total number of lone pairs:0
bond angle: 120/90
trigonal bipyramidal
total number of electron pairs: 5
total number of bonding pairs: 4
total number of lone pairs:1
bond angle: 119/89
trigonal pyramidal or see saw
total number of electron pairs: 5
total number of bonding pairs: 3
total number of lone pairs:2
bond angle: 120 or 89
trigonal planar or T-shape
total number of electron pairs: 6
total number of bonding pairs: 6
total number of lone pairs:0
bond angle: 90
octahedral
total number of electron pairs: 6
total number of bonding pairs: 5
total number of lone pairs:1
bond angle: 89
square pyramid
total number of electron pairs: 6
total number of bonding pairs: 4
total number of lone pairs:2
bond angle: 90
square planar
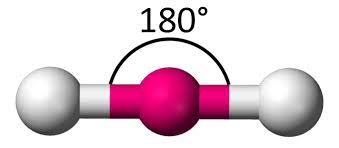
name this molecule
linear
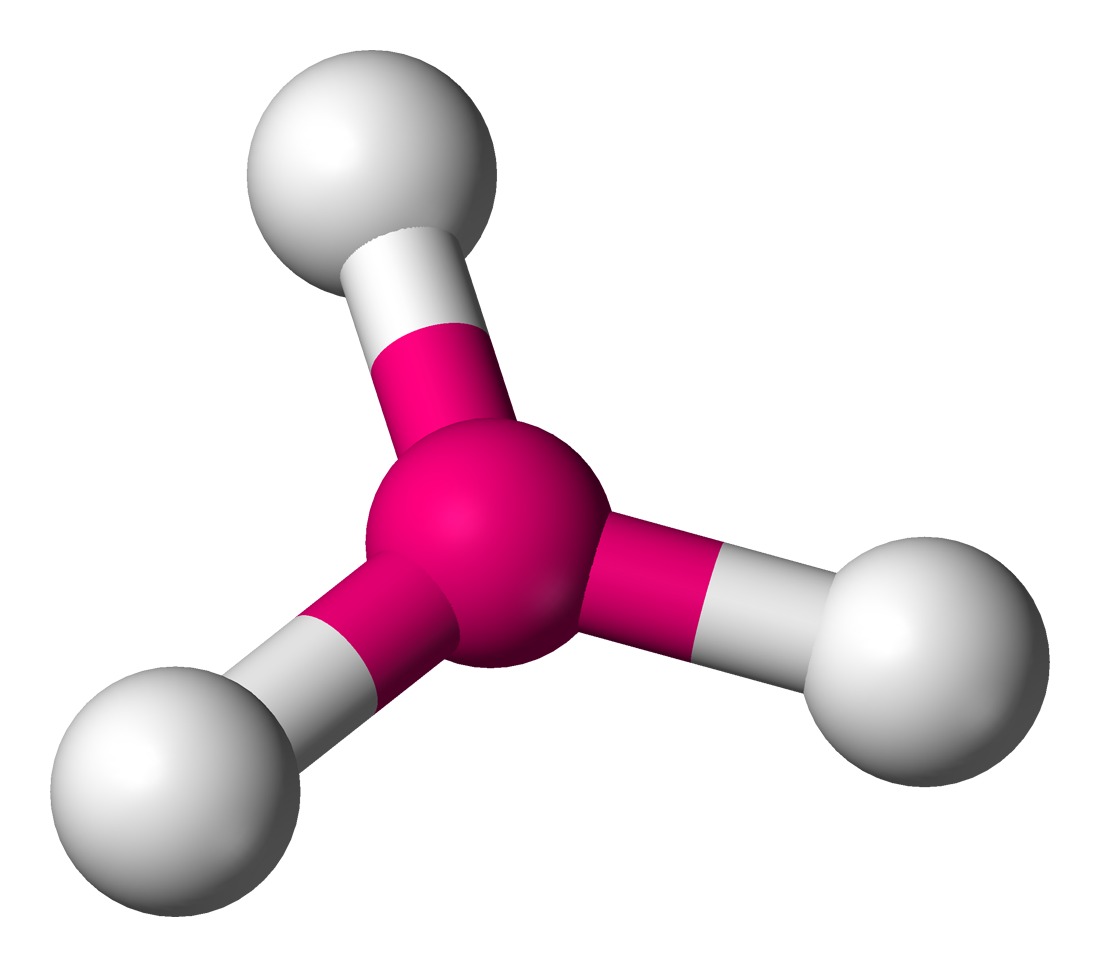
name this molecule - angle: 120
trigonal planar
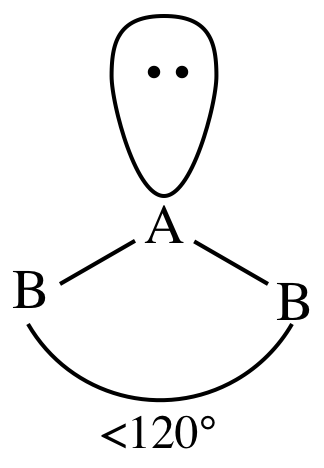
name this molecule - angle: 118
bent/v-shape
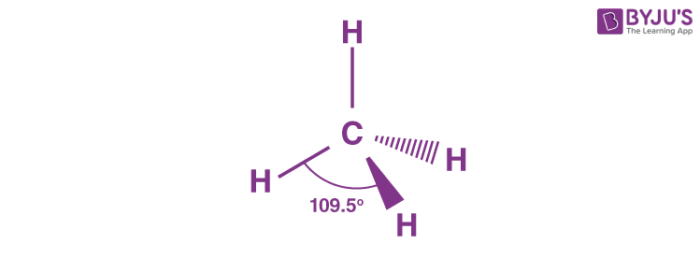
name this molecule
tetrahedral
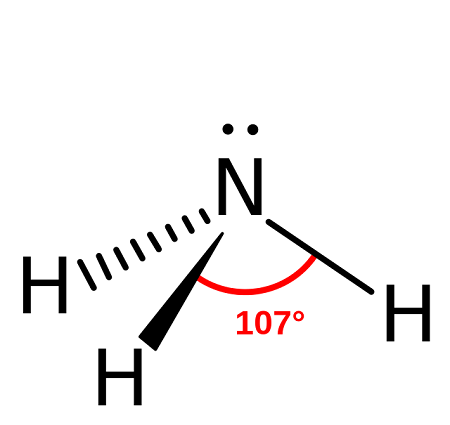
name this molecule
trigonal pyramidal
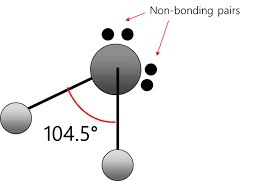
name this molecule
bent/ v-shape
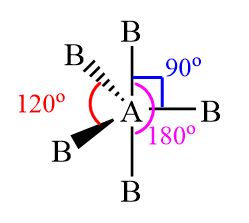
name this molecule
trigonal bipyramidal
name this moleculeangle: 119/89
see-saw
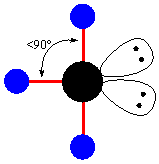
name this molecule - angle:89
t-shape
name this molecule
angle: 90
octahedral
name this molecule
angle: 89
square pyramid
name this molecule
angle : 90
square planar