CHAPTER 5: MACROMOLECULES
1/103
Earn XP
Description and Tags
by: hanna and jasmine :D
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
104 Terms
Synthesis and Breakdown of polymers
Facilitated by enzymes
Assembled by dehydration synthesis
Disassembled by hydrolysis
Condensation reaction
Reaction that connects a monomer to another monomer or a polymer
Two molecules are covalently bonded to each other with the loss of a small molecule
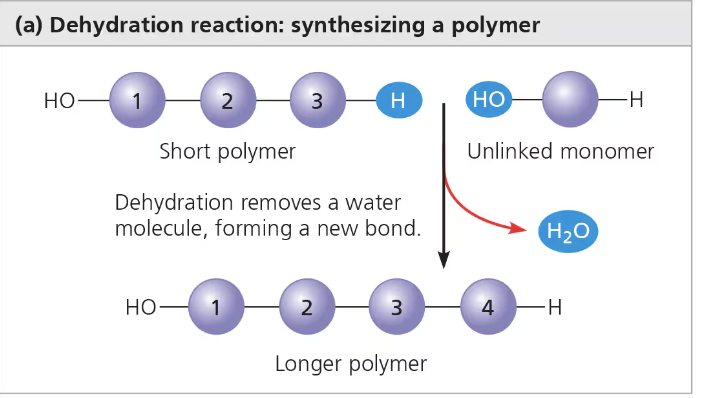
Dehydration Reaction/Synthesis (condensation)
Water is lost
Anabolic - building into larger (more complex) molecules
Increases complexity
Requires energy (endergonic) & enzymes
Produces water molecules
Ex. Carbs and protein polymers are synthesized by dehydration reactions
Each reactant contributes part of the water molecule that is released during the reaction
One provides a hydroxyl group (—OH), while the other provides a hydrogen (—H)
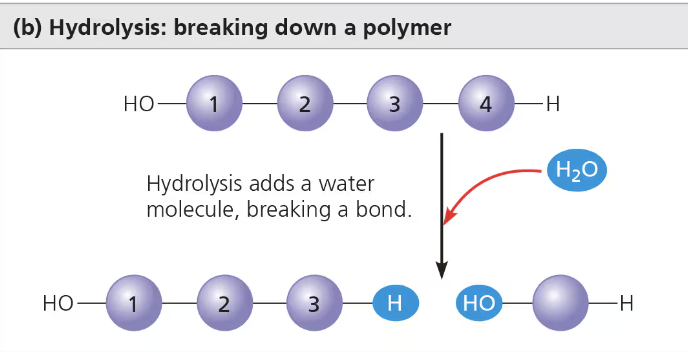
Hydrolysis
Polymers are disassembled
“lysis” - breaking
Catabolic
Reduces complexity
Water is needed as an input
Releases energy when bond is broken (exergonic)
Bond between monomers is broken by the addition of a water molecule
Ex. Process of digestion: bulk of the organic material in our food is in the form of polymers that are much too large to enter cells, in digestive tract, various enzymes attack the polymers, speeding up hydrolysis.
Carbohydrates
C H O
A source of energy and provide structural support.
Serve as fuel and building material
Simplest forms are the monosaccharides, or simple sugars (monomers)
Disaccharides are double sugars consisting of two monosaccharides joined by covalent bonds (glycosidic linkage)
Polymers are polysaccharides composed of many sugar building blocks.
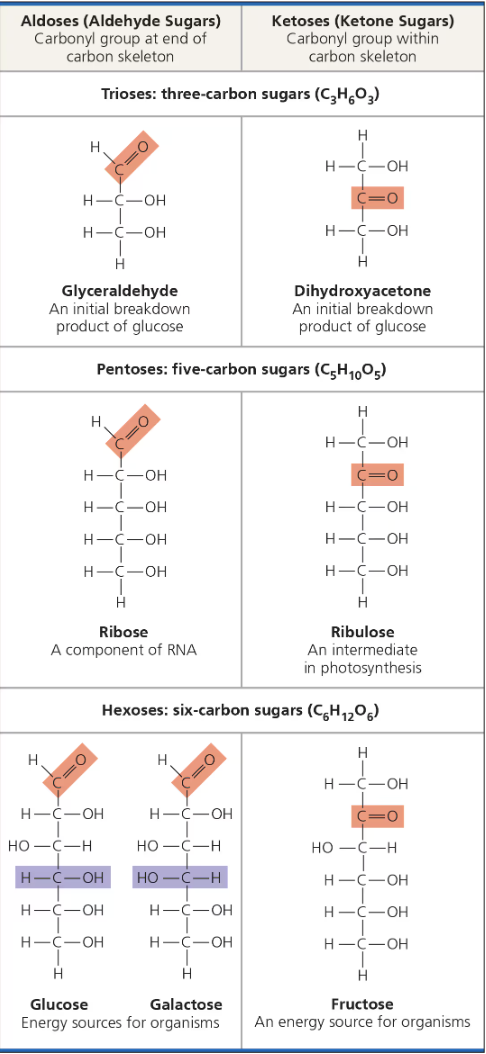
Monosaccharides (structure)
Generally have molecular fomulas that are some multiple of the unity CH2O.
Most common monosaccharide is glucose (C6H12O6)
Trademarks of a monosaccharide: carbonyl group, and multiple hydroxyl groups
Depending on location of carbonyl group, a monosaccharide is either an aldose (aldehyde sugar) or a ketose (ketone sugar)
Most name for sugars end in -ose. Ex. Glucose, galactose, fructose, ketose, aldose, etc.
Another criterion for classifying monosaccharides is its carbon skeleton which ranges from three to seven carbons long.
Monosaccharides (function)
Major nutrients for cells
Major fuel for cell work and their carbon skeletons serve as raw material for teh synthesis of other types of small organic molecules, such as amino acids and fatty acids
Disaccharide (structure)
Two molecules joined by glycosidic linkage (covalent bond formed between two monosaccharides by dehydration synthesis)
Ex. Maltose, disaccharide formed by linking of two molecules of glucose
Most prevalent dissacharide is sucrose w/two the two monomers glucose and fructose
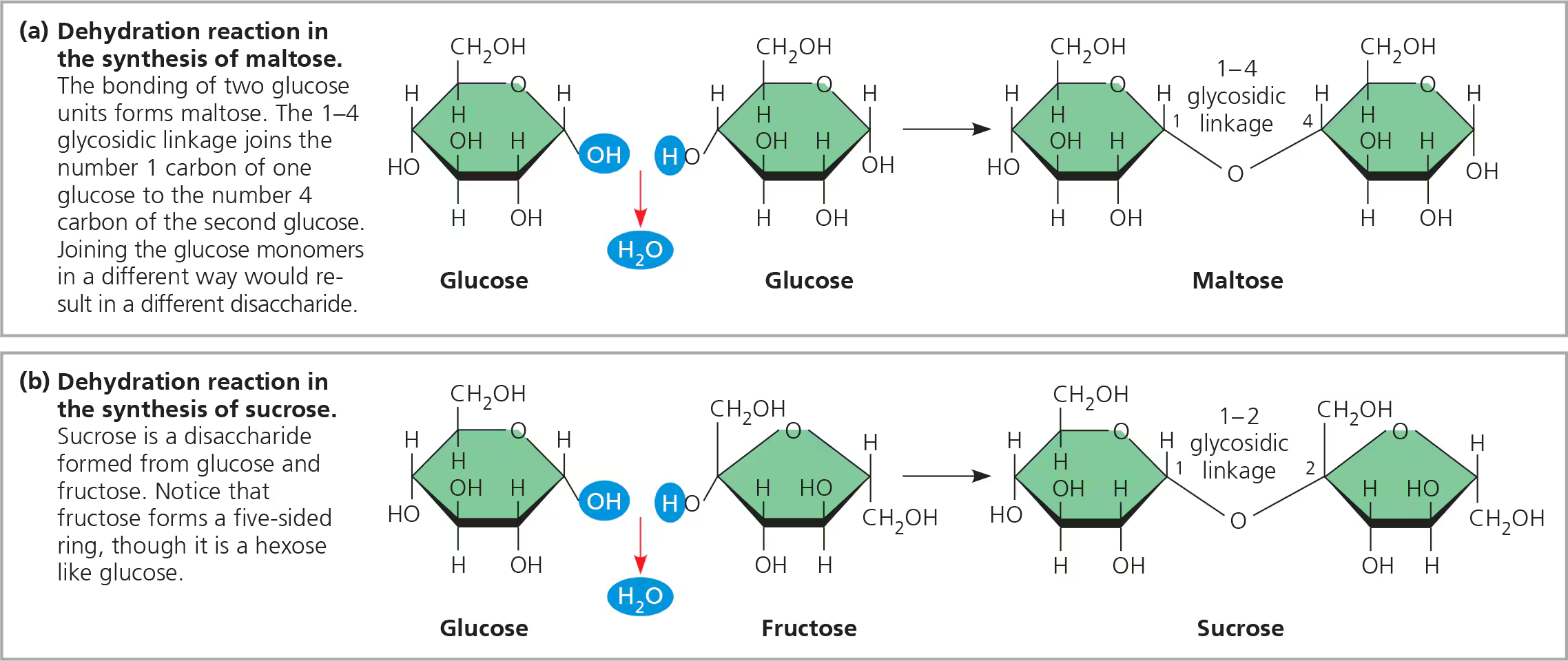
Disaccharide (function)
Must be broken down into monosaccharides to be used for energy by organisms
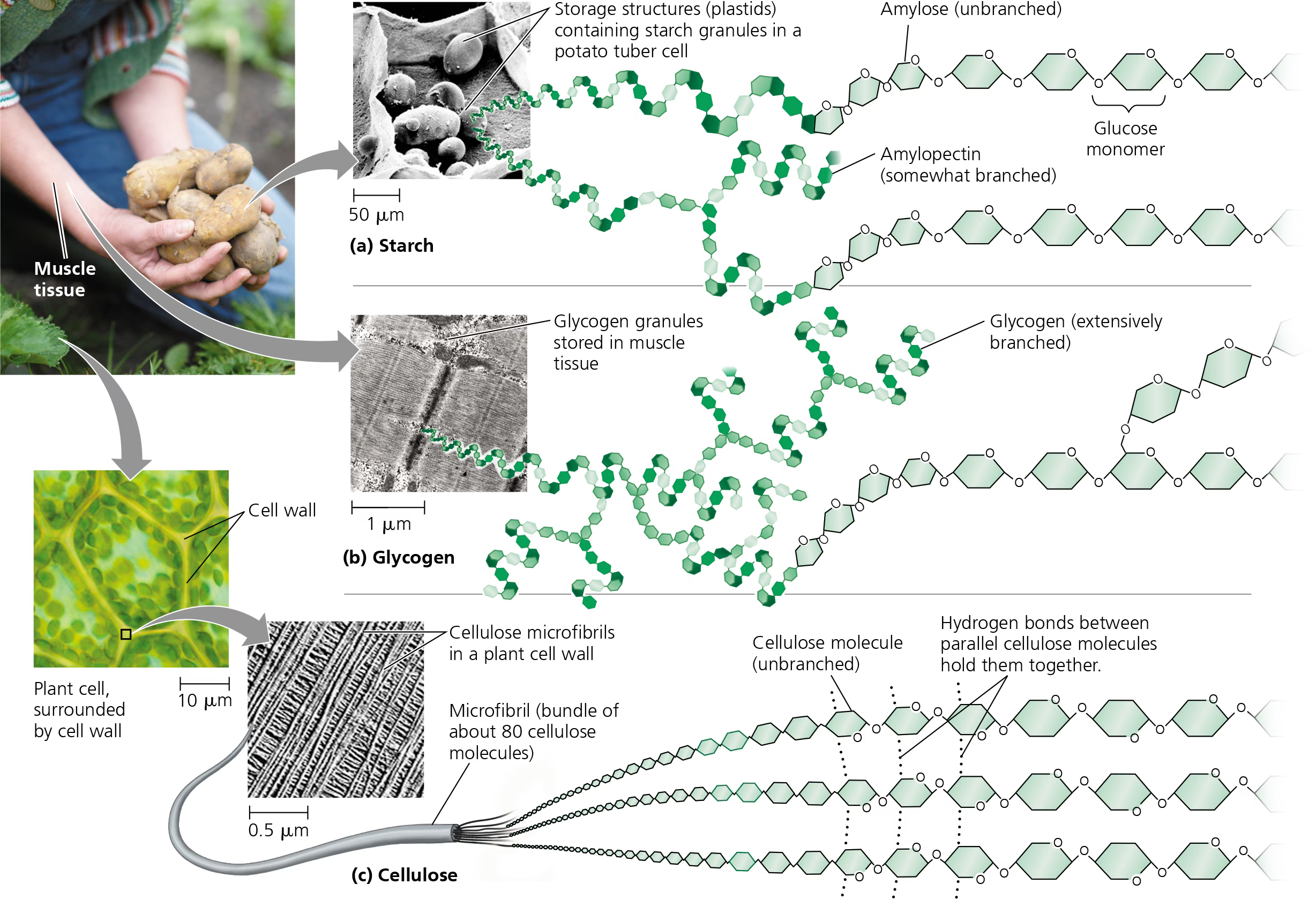
Polysaccharides
macromolecules, polymers w/ a few hundred to a few thousand monosaccharides joined by glycosidic linkages.
some polysaccharides serve as storage material, hydrolyzed as needed to provide monosaccharides for cells.
others serve as building materials for structures that protect the cell or the whole organism
architecture and function of a polysaccharide are determined by its monosaccharides and by the positions of its glycosidic linkages
they are NOT soluble in water & too large to pass through cell membrane
glycogen in animals in live and muscle cells
amylose or amylopectin (starch) in plants
Storage Polysaccharides
plants and animals store sugars for later use in the form of storage polysaccharidse
Plants store starch (polymer of glucose monomers), as granules within cellular strctures know as plastids
synthesizing starch enables the plant to stockpile surplus glucose
can later by withdrawn by hydrolysis
most animals, including humans have enzymes that can hydrolyze plant starch
Structural Polysaccharides
Organisms build strong materials from structual polysaccharides
Ex. Cellulose is a major component of the tough walls the enclose plant cells
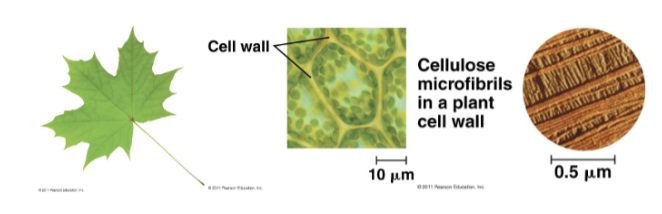
Difference between Starch and Cellulose
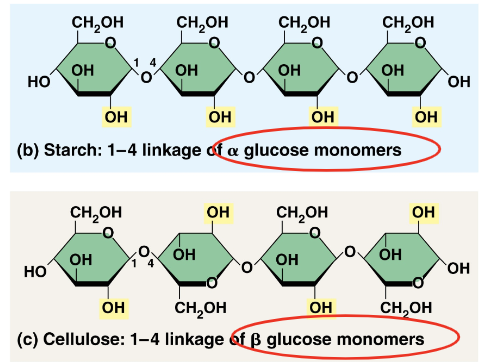
Linkages in two polymers differ w/ slightly different ring structures for glucose
When glucose forms a ring, hydroxyl group attached to the number 1 carbon is positioned either below or above the plane of the ring
The two rings for the glucose are called alpha and beta, respectively.
Starch’s glucose are in the alpha configuration, and cellulose’s glucose are in the beta configuration, making ever glucose monomer “upside down”
Other Carbs
Chitin: a derivative of glucose used in cell walls of fungi, arthropod skeletons, and dissolving stitches
Similar to cellulose with beta linkages
Peptidoglycan: a derivative of glucose used in bacterial cell walls
Lipids
Only macromolecule to not include true polymers
Grouped with each other b/c they are hydrophobic
Consists mostly of hydrocarbon regions with relatively polar C—H bonds.
Major function is energy storage
A gram of fat stores more than twice as much energy as a gram of a polysaccharide.
Plants function with bulky energy in the form of starch
Humans and other mammals stock their long-term food reserves in adipose cells which swell and shrink as fat is deposited and withdrawn from storage
Adipose cells also cushion such vital organs as the kidneys, and a layer of fat beneath the skin insulates the body
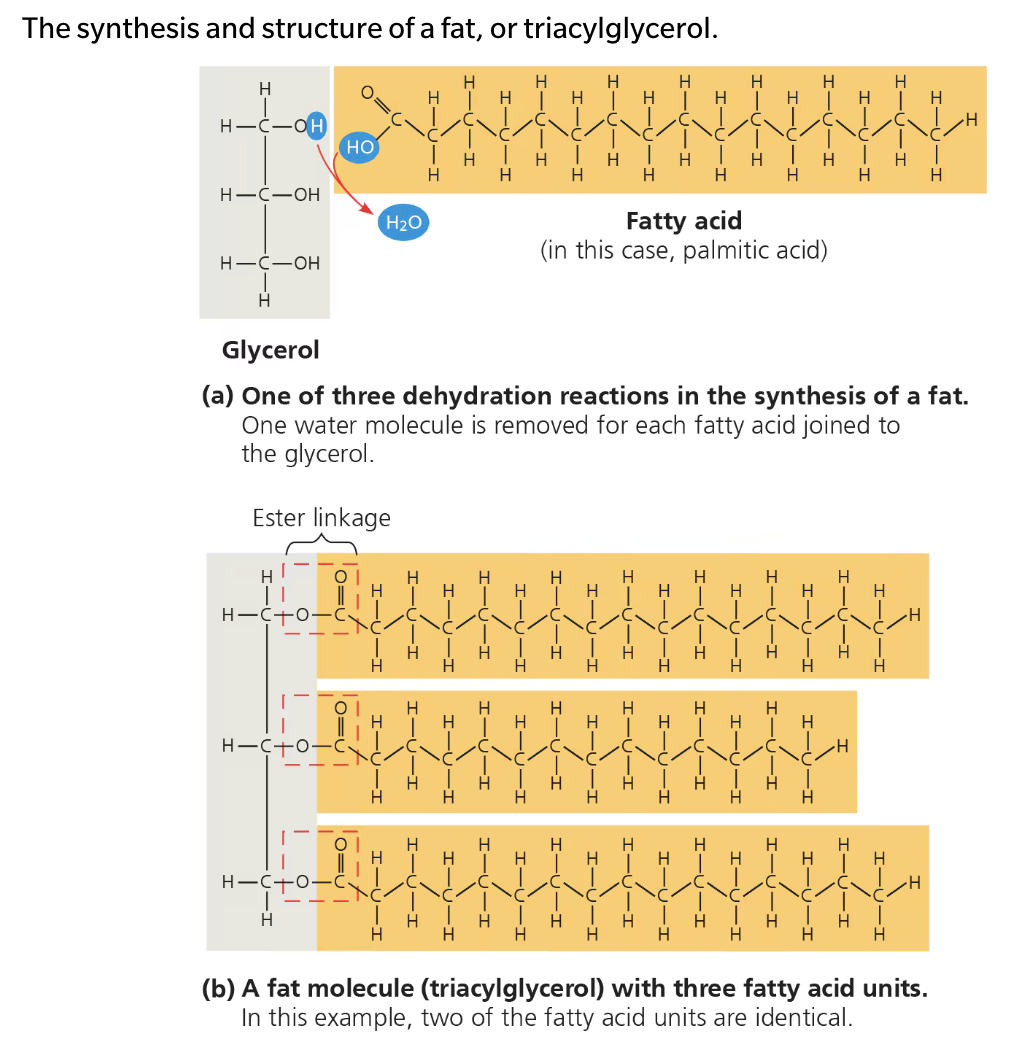
Fats (triglyceride)
Large molecules assembled from smaller molecules by dehydration synthesis
Consists of a glycerol molecule joined to three fatty acids
Glycerol is an alcohool; each of its three carbons bears a hydroxyl group
Fatty acid has a long carbon skeleton, ususally 16 or 18 carbon atoms in length
Carbon at one end of the skeleton is part of a carboxly group, gives the name fatty acid.
Rest of skeleton consists of hydrocarbon chain
Non polar C—H in hydrocarbon makes fats hydrophobic
Connected by ester linkage, via dehydration synthesis (bond between a hydroxyl group and carboxyl group)
Saturated fats (triglycerides)
No double bonds between carbon atoms composing a chain.
As many hydrogen atoms as possible are bonded to the carbon skeleton
Solid at room temp
Typically found in animals
Called fats
Too much contributes to plaque buildup in arteries
Ex. butter, lard, fat drained off from meat
Flexibility allows the fat molecules to pack together tightly
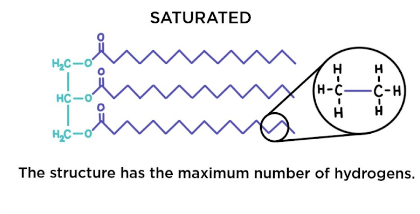
Unsaturated fats (triglycerides)
One or more double bonds
One fewer hydrogen atom on each double-bonded carbon
Liquids at room temp
Called oils
Can be mono or polyunsaturated
One double bond is most common
2 or more double bonds are more rare
Typically found in seeds & provides energy to growing plants
Ex. olive oil, sunflower oil
The kind where the cis double bonds are located prevent the molecules from packing together closely enough to solidify at room temp.
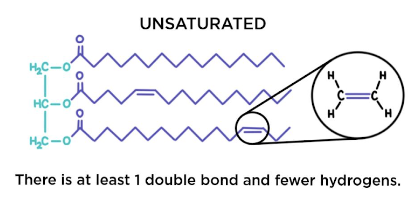
Cis-isomers
very common in nature
the hydrogen atoms are on the same side of the two carbon atoms
the double bond causes a bend in the fatty acid chain
the refore cis-isomers are only loosely packed
Triglycerides formed from cis-isomers have melting points - they are usually liquids at room temp.
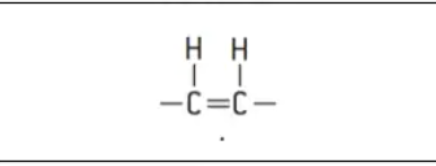
Trans-isomers
Rare i nature - usually artificially produced to produce solid fats, e.g. margarine from vegetable oil
the hydrogen atoms are on different sides of the two carbon atoms
the double bond does not cause a bend in the fatty acid chain
trans-isomers can be closely packed
triglycerides formed from trans-isomers have melting ponts - usually solid at room temp.
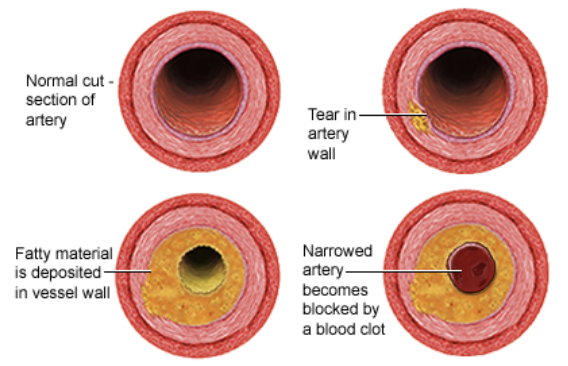
Atherosclerosis
Diet rich in saturated fats contribute to the cardiovascular disease know at atherosclerosis
Deposits called plaques develop within the walls of blood vessels, causing inward bulges that impede blood flow and reduce the resilience of the vessels
Phospholipids
Essential for cells because they are major constituents of cell membranes
Their structure provides a classical example of how form fits function at the molecular level
Has a hydrophilic (polar) head and two hydrophobic (nonpolar) tails
Only has two fatty acids attached to glycerol rather than three
The third hydroxyl group of glycerol is joined to a phosphate group
Typically, an additional small charged or polar molecule is also linked to the phosphate group
Ex. Choline
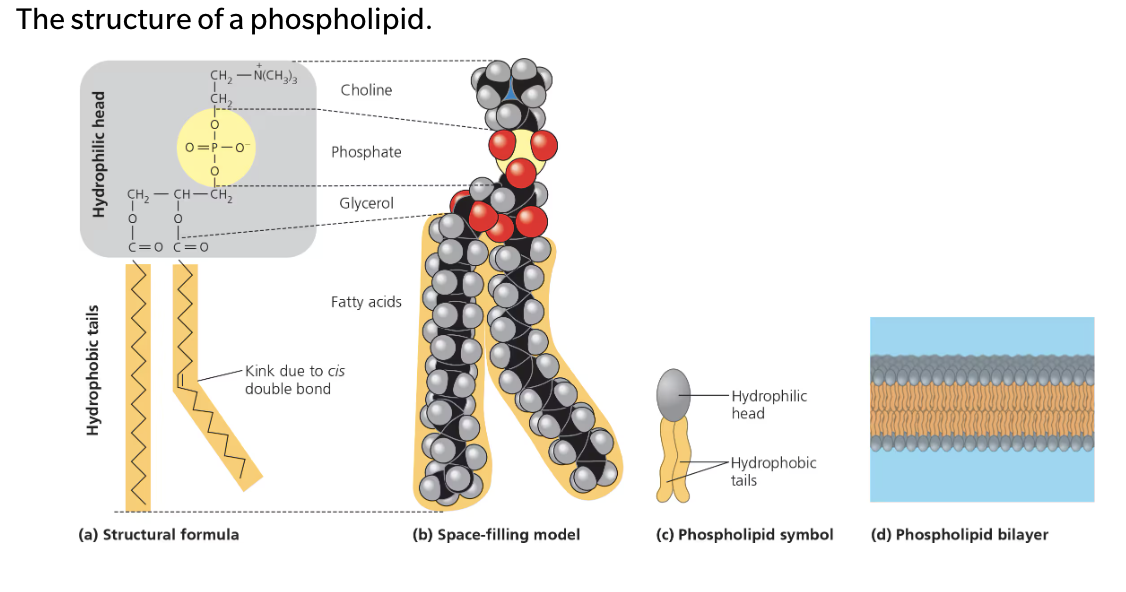
Phospholipid bilayer
Shields the hydrophobic fatty acid tail from water
Hydropholic heads of the molecules are on the outside of the bilayer, in contact with the aqueous solutions inside and outside of the cell.
The hydrophobic tails point toward the interior of the bilayer, away from the water
The bilayer foarms a boundary between the cell and its external enviorment and established seperate compartments within eukaryotic cells.
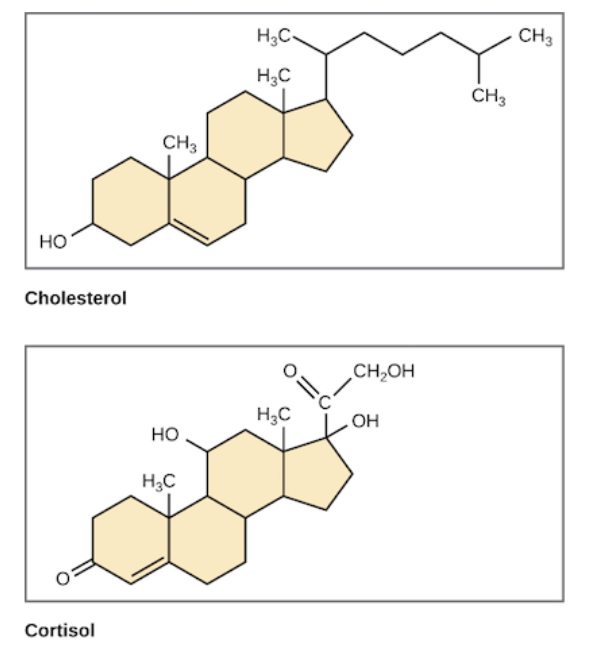
Steroids
Lipids characterized by a carbon skeleton consisting of four fused rings
Distinguished by the particular chemicals groups attached to the ensemble of rings
Some may have a hydrocarbon tail
Ex. Cholesterol: Cruicial to animls
Common component of animal cell memberans and also the percursor from whihch other steroids, such as the vertebrae sex hormones, are synthesized
High level may contribute to atherosclerosis
Write the formular for a monosaccharide that has three carbons
C3H6O3
A dehydration reaction joins two glucose molecules to form maltose. The formula for glucose is C6H12O6. What is the formula for maltose?
C12H22O11
WHAT IF? After a cow is given antibiotics to treat an infection, a vet gives the animal a drink of “gut culture” containing various prokaryotes. Why is this necessary?
The antibiotic treatment is likely to have killed the cellulose-digesting prokaryotes in the cow’s gut. The absence of these prokaryotes would hamper the cow’s ability to obtain energy from food and could lead to weight loss and possibly death. Thus, prokaryotic species are reintroduced, in appropriate combinations, in the gut culture given to treated cows.
Compare the structure of a fat (triglyceride) with that of a phospholipid/
Both have a glycerol molecule attached to fatty acids. The glycerol of a fat has three fatty acids attached, whereas the glycerol of a phospholipid is attached to two fatty acids and one phosphate group.
Why are human sex hormones considered lipids?
Human sex hormones are steroids, a type of compound that is hydrophobic and thus classified as a lipid.
WHAT IF? Suppose a membrane surrounded an oil droplet, as it does in the cells of plant seeds and in some animal cells. Describe and explain the form it might take.
The oil droplet membrane could consist of a single layer of phospholipids rather than a bilayer, because an arrangement in which the hydrophobic tails of the membrane phospholipids were in contact with the hydrocarbon regions of the oil molecules would be more stable.
polymerization
chemical merchanims by which cells make polymers; facilitated by enzymes
polymer
long molecule consisting of many similar identical building blocks linked by covalent bonds
macromolecules
basis of complex cellular life
single molecules that consists of many covalently linked single units
small units of monomers linked together to form polymers
monomer
building block unit of a polymer
enzymes
specialized macromolecules (proteins) that speed up chemical reactions
condensation reaction
a reaction in which two molecules are covalently bonded to each other without loss of small molecules
dehydration synthesis
a process in which two monomers/molecules are covalently bonded with the loss of a water molecule
anabolic
increases complexity
requires energy (endergonic) and enzymes
produces water molecules
type of condensation reaction
hydrolysis
a process where polymers are disassembled into monomers by adding a water molecule
enzymes speed up hydrolysis
catabolic (breaking)
reduces complexity
water is needed as an input
releases energy when bond is broken (exergonic)
ex: process of digestion (breaking down starch and other carbs)
carbohydrates
monomer: monosaccharide [ratio is 1:2:1]
polymer: polysaccharide (ex: glycogen/starch)
type of bond/linkage: glycosidic linkage via dehydration synthesis
biological function:
short term energy
structural support
cell-to-cell communication
arrangement: around asymmetric carbon; ring-shape skeletons are most stable in aqueous solution (5-6 carbons)
aldose
aldehyde sugar (ex: glucose)
ketose
ketone sugar (ex: fructose)
oligosaccharide
3-10 carbon sugar molecules
hexoses
6 carbon sugars (ex: glucose, fructose)
tricoses
3 carbon sugars (ex: glycealdehyde)
pentoses
5 carbon sugars (ex: ribose and ribulose)
disaccharide
two monosaccharides joined by a covalent bond (glycosidic linkage)
ex: table sugar/sucrose (glucose + fructose), maltose (glucose + glucose), lactose (glucose + galactose)
cellulose
strengthens plant cell walls
starch
stores glucose for energy in plants (ex: amylose/amylopectin)
a polymer of glucose monomers, as granules within cellular structures known as plastids
plants withdraw starch using hydrolysis
amylose: most simplest form of starch; unbranched
glycogen
stores glucose for energy in animals (in liver and muscle cells)
polymers off glucose that is extensively branched
in humans, glycogen stores are depleted in about a day, which raises concern for low carb diets
extensive branch allows for its free ends to break down for energy
chitin
strengthens animal exoskeletons and fungal cell walls
problem of herbivory
herbivores need to digest cellulose from the plants that they eat, but animals lack the enzymes necessary to break down the beta linkages in cellulose
ex: termites cannot break down cellulose, so they have developed a symbiotic relationship with a protist (in exchange of the protist living in the termite’s gut, the protist does the cellulose digestion)
ruminants
animals, such as cows, that have a vastly expanded upper GI tract; cellulose is digested from bacteria and continual regurgitation/chewing of the “cud”
caecophores
animals, such as bunnies, with an expanded lower GI tract
food cannot be regurgitated (cellulose is only partially digested), but it can be digested further by re eating their partially digested cellulose (they eat their poop)
peptidoglycan
a derivative of glucose used in bacterial cell walls (carbs)
lactose intolerance
common condition in humans who lack lactase, the enzymes that breaks down lactose
instead, lactose is broken down by the intestinal bacteria in your gut, which causes cramping and gas
polysaccharide
polymers with 100+ monosaccharides joined by glycosidic linkages
structure and function is determined by its monosaccharides and positions of its glycosidic linkages
lipids
elements: CHO
monomers:
triglyceride - glycerol + 3 fatty acid chains
phospholipids - glycerol + 2 fatty acid chains + phosphate group
steroids - 4 ringed structures; lipids characterized by a carbon skeletons consisting of four rings
polymer: none
type of bond: ester bond via dehydration synthesis
biological functions: long term energy source, makes up cell membrane (phospholipids), insulation, hormones
ex: fats, oils, waxes
triglyceride
glycerol and 3 fatty acids
phospholipid
glycerol, 2 fatty acid chains, and a phosphate group
ampipathic
third hydroxyl group of glycerol is joined to a phosphate group (ex: choline)
when in contact with water, they self-assemble into a double layer sheet called “bilayer” that shields the hydrophobic fatty acid tails from water
steroids
lipids characterized by a carbon skeletons with cortisol, and consists of four fused rings
ex: cholesterol, estrogen, testosterone and some lipid hormones
some may have a hydrocarbon tail
functional groups give different characteristics
characteristics of lipids
hydrophobic (hates water)
mixes poorly due to molecule structure
hydrocarbon regions w/ non-polar C-H bonds
characteristics of saturated fatty acids
solid at room temperature
typically found in animals
called fats
too much contributes to plaque buildup
ex: butter, fat drained from meat, lard
characteristics of unsaturated fatty acids
liquid at room temperature
called oils
can be mono or polyunsaturated
one double carbon bond is most common
2 or more double bonds are rare
**usually are cis-isomer bonds
typically found in seeds and provide energy to growing plants
ex: olive oil, sunflower oil
what are the three parts of the phospholipid layer
hydrophilic head (polar), hydrophobic tail (non polar), and the phospholipid bilayer (essentially everything)
wax
long chain of fatty acid bonds to a long chain alcohol
plants use: serves as protective covering + slows water loss
maintains skin/fur, traps dust/dirt, and form honeycombs
ampipathic
has both polar and non polar regions
cholesterol
type of steroid that helps maintain fluidity in cell membrane; too much can cause plaque buildup in arteries
nucleic acid
elements: CHONP
monomers: nucleotide (one pentose, nitrogenous base, 1-3 phosphate groups)
polymers: polynucleotide
type of bond: phosphidester bond via dehydration synthesis
biological function:
carry genetic blueprint
gene expression
carrying instructions from DNA to ribosomes (RNA)
protein synthesis
energy
nucleotide
has one pentose, a nitrogenous base, and 1-3 phosphate group
pyrimidine
nitrogenous base w/ one six-membered ring of carbon and nitrogen atoms (CTU)
has one carbon ring (ex: cytosine, uracil, thymine)
purine
six membered ring fused to a five-membered ring
has two carbon rings (ex: adenine and guanine | AGGIES ARE PURE)
what bonds hold the two DNA chains together?
hydrogen bonds
mRNA
messenger RNA that interacts with the cell’s proteins, synthesizing machinery to direct production of polypeptides
what is the flow of genetic info?
DNA → RNA → protein
ribosome
site of protein synthesis
antiparallel (in DNA)
two sugar phosphate backbones are outside the helix while nitrogenous bases are in the interior of the double helix
tRNA
transfer RNA that brings amino acids to the ribosome during the synthesis of a polypeptide
DNA sequencing
determining the sequence of nucleotides along a DNA strand
nucleoside
molecule that only has the pentose and nitrogenous base of a nucleotide (NO PHOSPHATE GROUP)
genome
the entire sequence of the full component of DNA
human genome project goal
goal was to sequence the entire human genome
benefits: rapid development of faster and cheaper methods of sequencing
bioinformatics
the use of computer software and other computational tools that can handle and analyze large data sets
genomics
analyzing and comparing genomes of different species
proteomics
analysis of large sets of proteins
protein sequences → found by using biochemical techniques of translation of DNA sequence
linear sequences in DNA (nucleotides) can determine amino acid sequence of proteins
proteins
elements: CHON
monomers: amino acid
polymer: polypeptide
type of bond: peptide bond via dehydration synthesis
biological function:
catalyze chemical reactions
protect against disease
store amino acids
transport substances
provides structural support
receives signals from outside cell
functions in cell movement
ex: hemoglobin, lactase, growth hormones, other enzymes
catalysts
chemical agents that selectively speed up chemical reactions w/o being consumed in the reaction
protein (definition)
a biologically functional molecule made up of one or more polypeptides coiled in a specific 3D structure
primary structure
sequence of amino acids in one polypeptide chain
formed via peptide bonds
secondary structure
repeating 3D structures found in all polypeptide chains
hydrogen bonding of the peptide backbone (H of amino group & oxygen of the carboxyl group) causes the amino acids to have a repeating pattern
R groups do NOT play a role (yet)
tertiary structure
three dimensional folding pattern of a polypeptide chain/protein due to side chain (R group) interactions
protein is now functional bc it is folded in 3D structure
structure bends in a certain way due to the interaction between R groups and the local environment of the cell
quaternary structure
specific 3D shape of a protein that contains more than one polypeptide chain, also known as subunits
NOT all proteins will be functional →due to the interactions between the subunits help to stabilize the overall structure
ex: sickle cell anemia; part of hydrophobic region of the RBC exposed will cause it to cave in
storage proteins
storage of amino acids
ex: casein (protein of milk); major amino acid source for baby mammals (also found in plant seeds)
ex 2: evalburmin (protein of egg white source for embryos)
hormonal proteins
coordination of an organism’s activities
ex: insulin, regulates blood sugar concentration
structural proteins
provides support
ex: keratin (hair), horns, feathers, skin appendages
collagen, elastin
defensive proteins
proteins that defend against infection
ex: antibodies
transport proteins
transports substances
ex: hemoglobin transport oxygen
receptor proteins
response of cell to chemical stimuli
ex: receptors built in membrane of a nerve cell detect signaling molecules of other nerve cells
contractile and motor proteins
provides movement
ex: motor proteins are responsible for cilia and flagella
acting and myosin are responsible for muscle contractions
enzymatic proteins
selective acceleration of chemical reactions
ex: digestive enzymes catalyze the hydrolysis of bonds in food molecules