CHEM 215 FINAL
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
Conjugated dienes
cis: same side
trans: opposite sides (more stable)
spirobicycles
2 rings connected by a single atom
Fused bicycles
2 rings that share 2 atoms
bridged bicycles
2 atoms that share more than 2 atoms
Diels alder reactions
are faster when 1 SM has an electron withdrawing group and the other has an electron donating group
Heat
the only necessary reagent for diels alder reactions
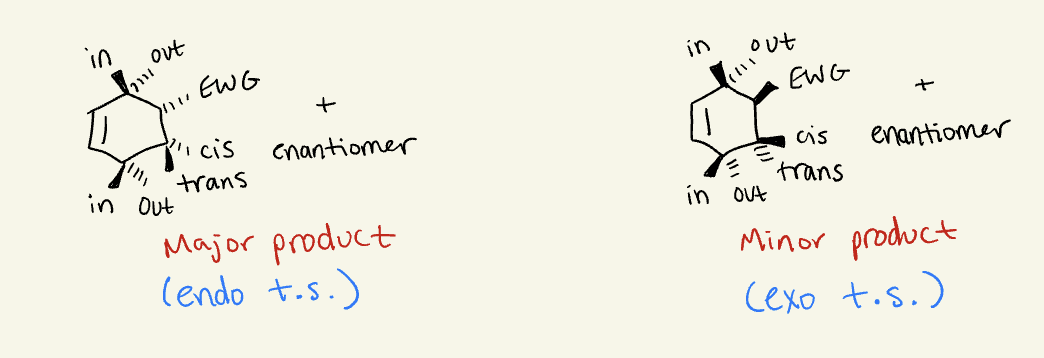
exo
substituent points to smaller bridge
endo
substituent points to bigger bridge
limitations to endo/exo
Can not be used for bridges that are the same size
type of reaction diels alder is
addition reaction
cycloaddition reactions
number of bonds formed
3 pi bonds → 1 pi + 2 sigma
types of dienes
isolated: sp3 carbon with no delocalized electrons
cumulative: sp hybridized carbon
conjugated: delocalization is possiblr
oxidation of aldehydes in carbohydrates
forms carboxylic acids
reagents of oxidation
AgNO3, NH3, Ag2O, NaOH
CuSO4, NaOH,
Br2,H2O
addition reactions of carbonyls reagents
-CH3OH/cat acid
- PhCh2SH/cat acid
-NaCN/H2O
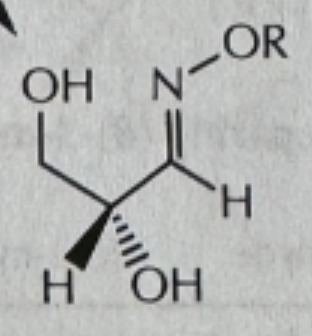
-RONH2/PH6
-NaBH4/CH3OH
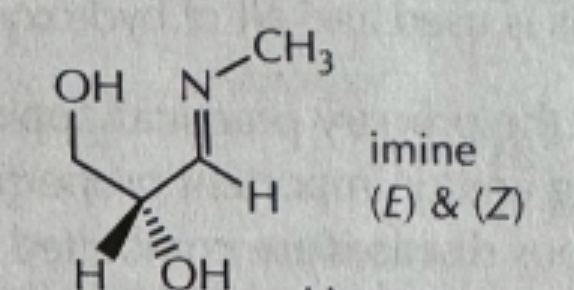
-CH3NH2/PH6
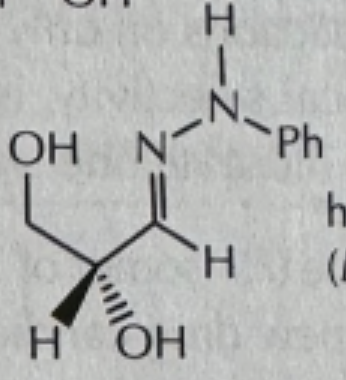
-PhNHNH2/PH6
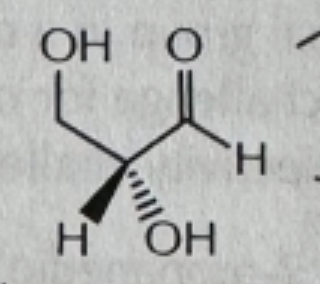
CH3OH/cat acid
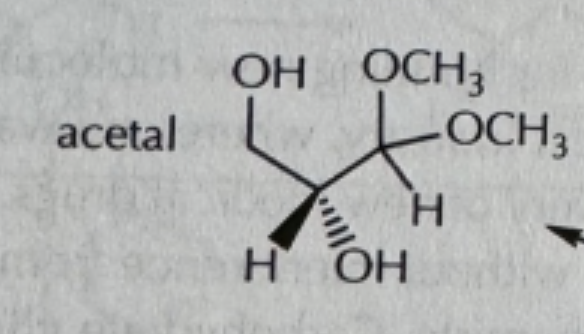
PhCh2SH/cat acid
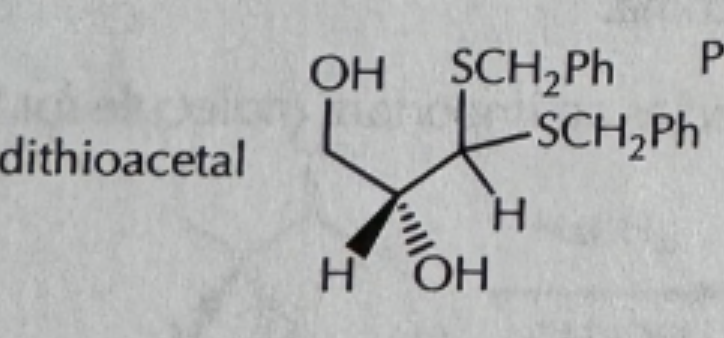
NaCN/H2O
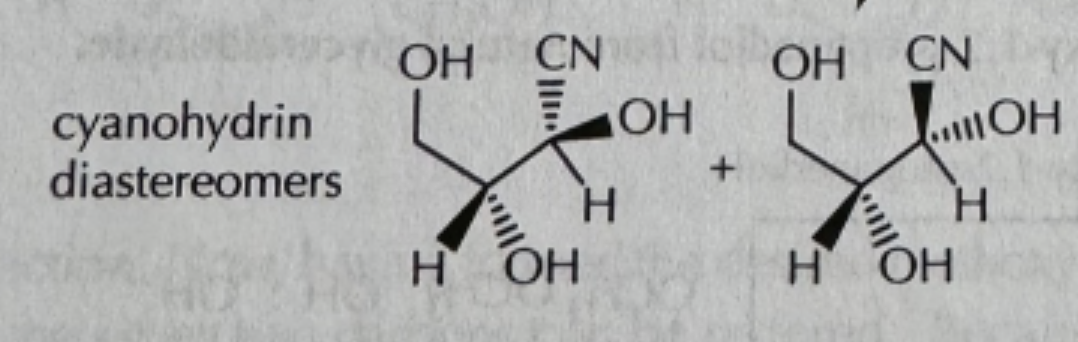
RONH2/PH6
NaBH4/CH3OH
CH3NH2/PH6
PhNHNH2/PH6
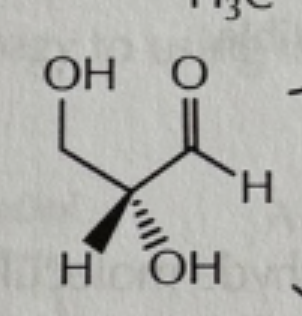
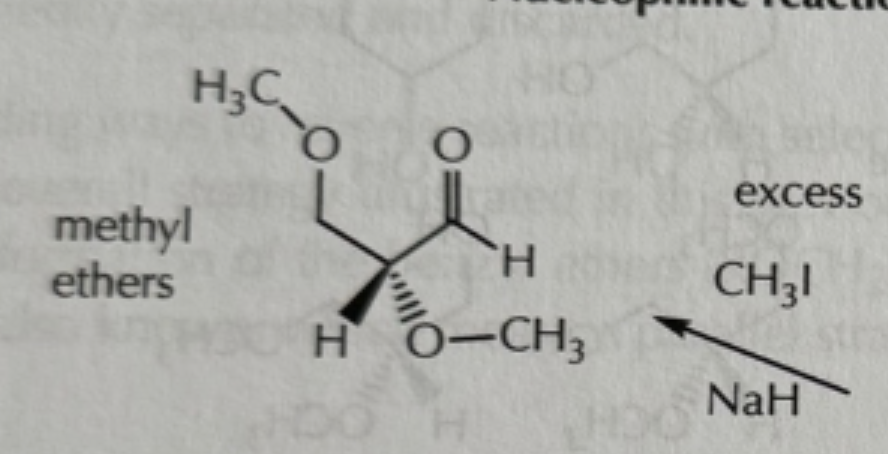
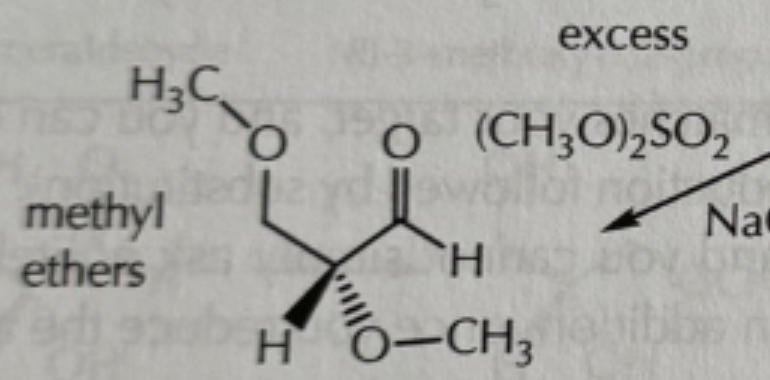
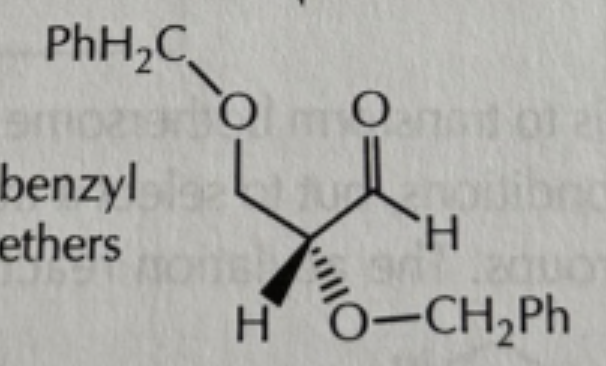
addition and acylation reactions of hydroxyls reagents
-everything is in excess!
-CH3I/NaH
-(CH3O)2SO2/NaOH
-PhCH2Br/NaH
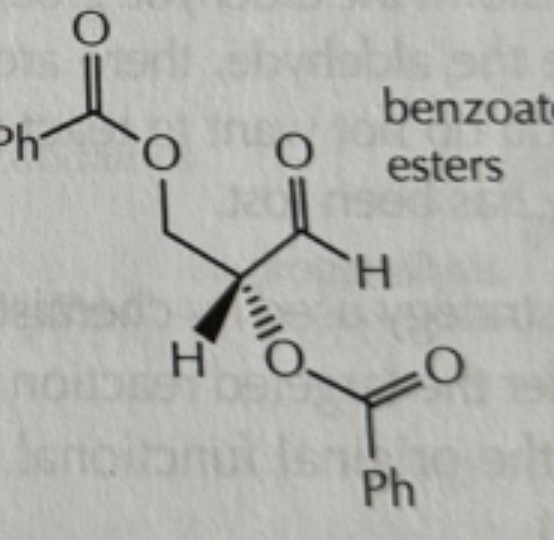
-PhCOCl/N(CH2CH3)3
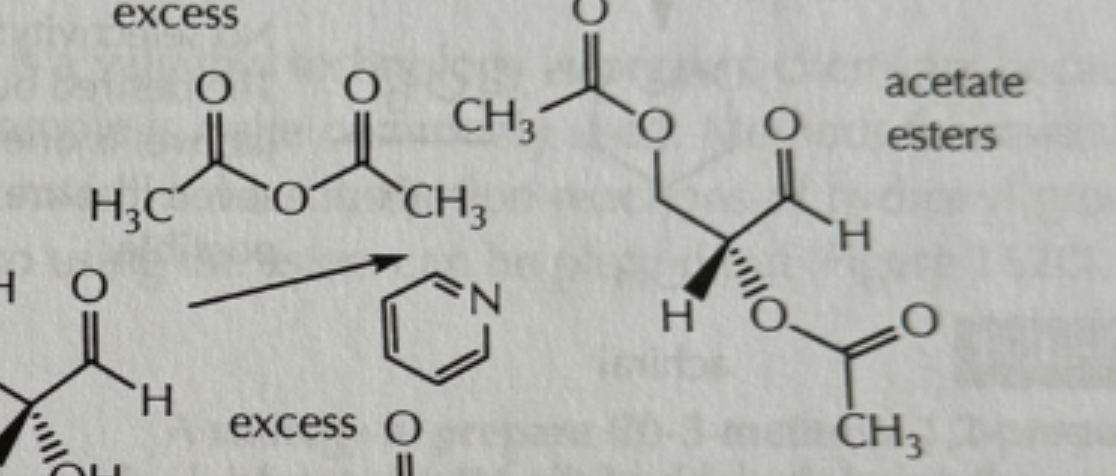
-CH3COOCOCH3/pyradine
CH3I/NaH
(CH3O)2SO2/NaOH
PhCH2Br/NaH
PhCOCl/N(CH2CH3)3
CH3COOCOCH3/pyradine
reducing sugar
must contain an aldehyde or hemiacetal
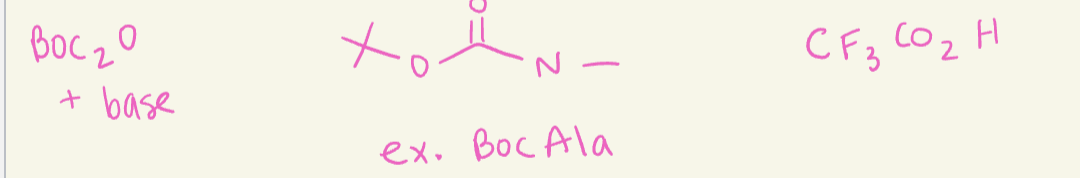
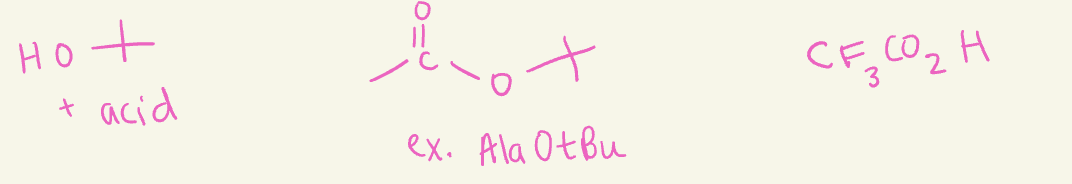
N-Terminus protecting groups
use base
BOC2O
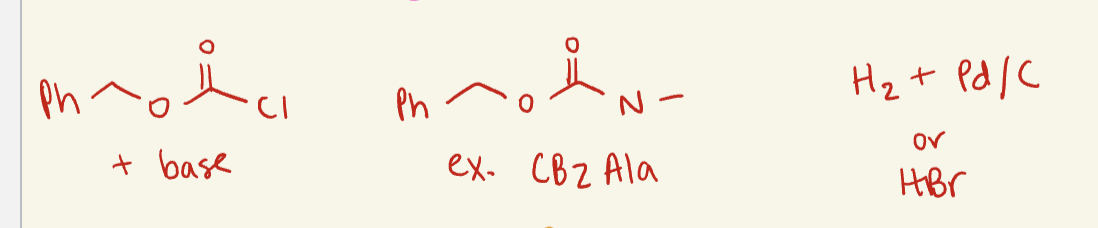
CBZ
FMOC
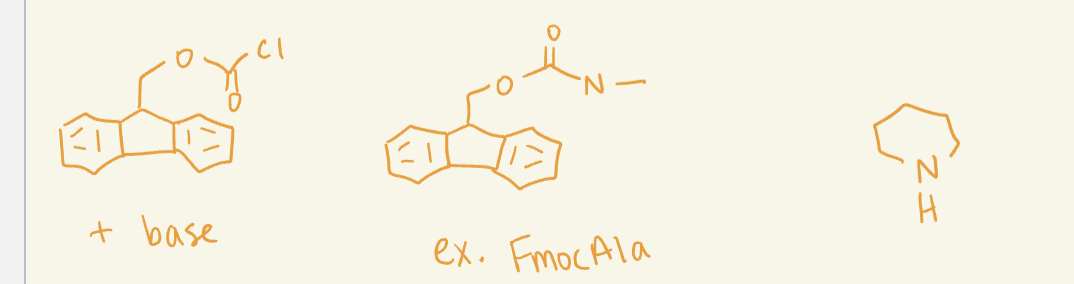
C-terminus protecting groups
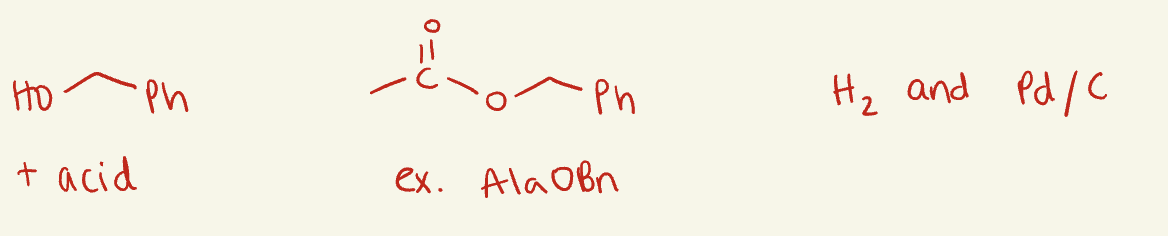
use acids (i.e H2SO4)
OtBu
OBn
OMe
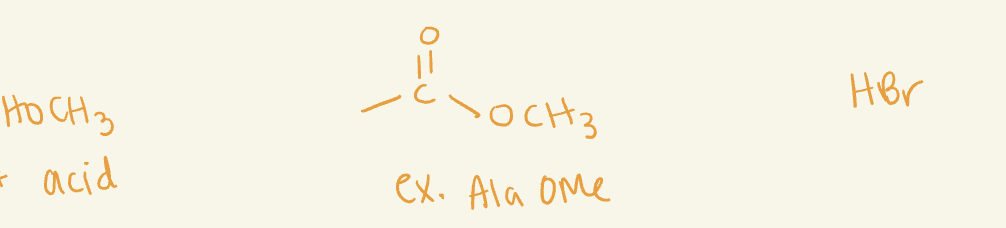
DCC
- acylating agent used to form peptide bonds
-helps add amino acids with protecting groups onto other amino acids
isoelectric point
Ph where the molecule carries no electrical charge
Products of diels alder
H2O/H2SO4
- can break acetals/ketals into mixed alcohols
-can not work on OCH2Ph unless it is an anomeric alcohol
CH3OH/H2SO4
-only works on anomeric alcohol
Jones
- creates carboxylic acids
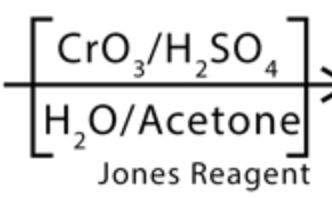
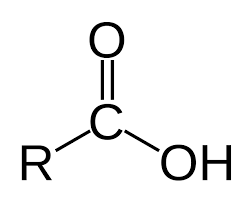
Carboxylic acid
-forms from primary alcohols with water
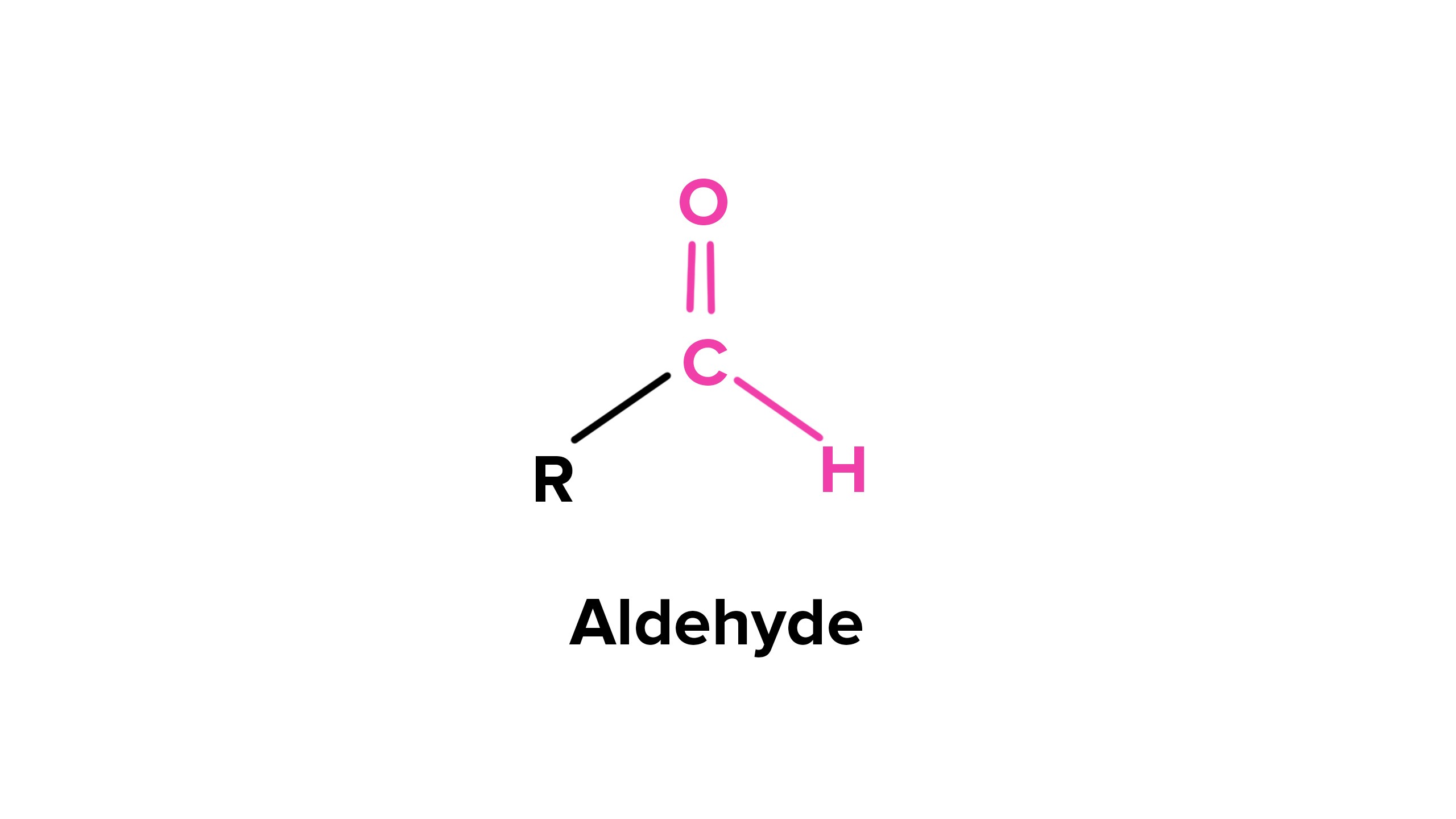
Aldehyde
- forms from primary alcohols without water
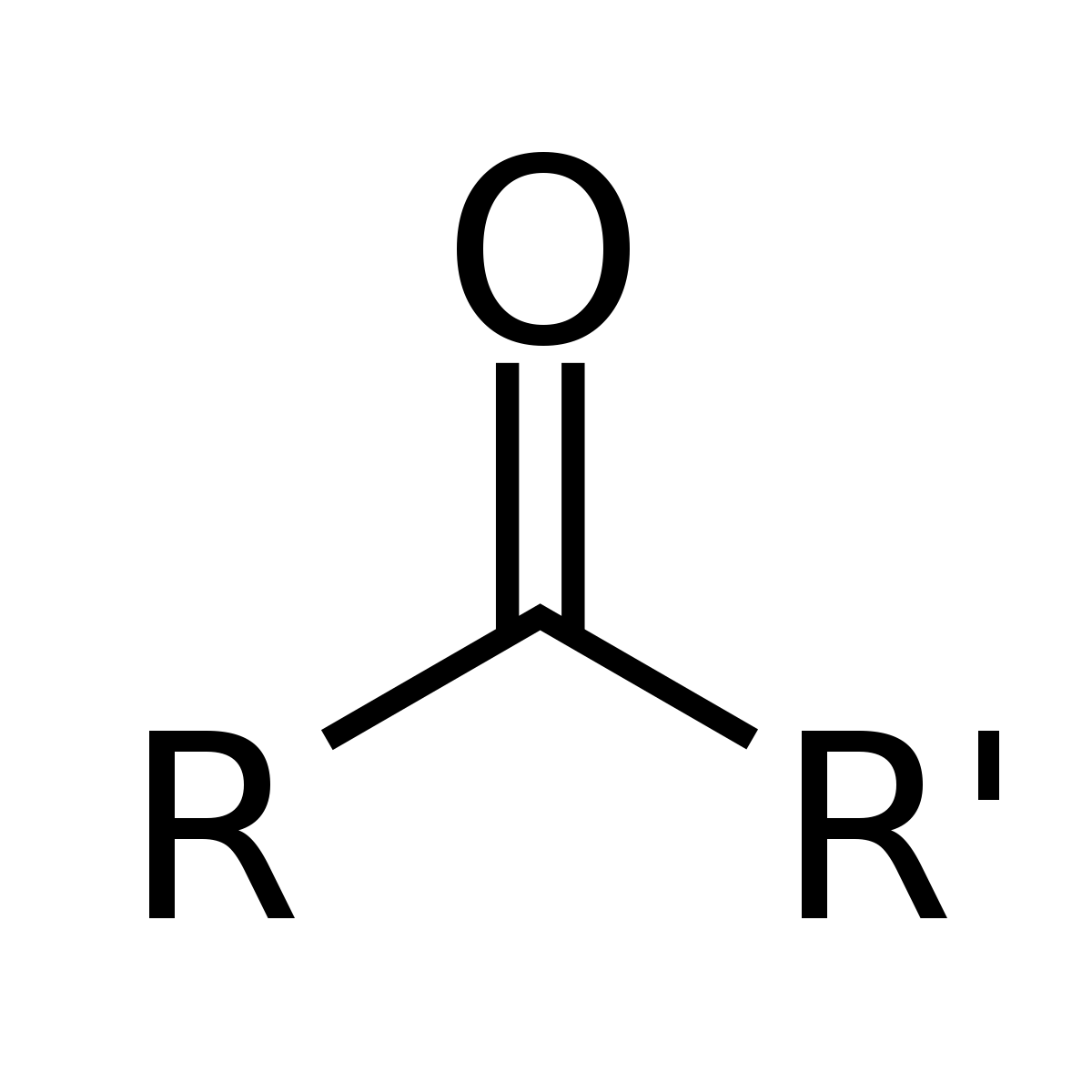
ketone
-forms from secondary alcohols
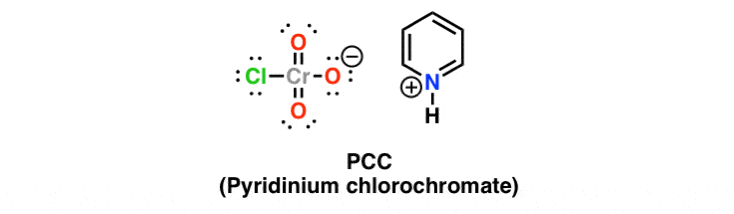
PCC
PDC
swern oxidation
Hydroboration
- anti-markonikov addition of H2O
Regio: anti-markonikov
Stereo: Syn
Adds: -OH and -H
Common reagents: BH3 or B2H6
Common solvent: H2O2, NaOH
Hydration
- markonikov addition of H2O
Regio: Markonikov
stereo: syn and anti
Adds: -OH and -H
Common reagents: H2O
Common solvents: H2SO4
Halogenation
anti-addition reaction
Regio: none
stereo: anti
adds: Br2, Cl2, I2, F2, etc.
Halohydrin
anti-additon reaction
regio: none
stereo: anti
adds: -OH and -X
common reagents: Br2+H2O , NBS + H2O, Br2+ROH, NBS + ROX
Hydrohalgenation
-addition to alkene
regio: Markonikov
stero: syn and anti
adds: -H and -X
Common reagents: HCl, HBr, HI
Dihydroxylation
Regio: none
stereo: syn
adds: -OH and -OH
Reagent: OSO4 or KMnO4
2nd step: NaOH,H2O or Na2SO3,H2O
Ozonolysis
Regio: none
Stereo: syn
common reagent: 1. O3
Common solvents: 2. Zn or 2.H2O2 or CH3CH3S
Epoxidation
regio: none
stereo: syn
common reagent: RCOOOH
Hydrogenation
- reduction reaction
regio: none
stereo: syn
adds: -H and -H
common reagent: H2
common solvent: Pd/ C
alkynes: H2 will add until fully saturated
Poised Catalyst
-reduction reaction
regio: none
stereo: syn
adds: -H and -H
common reagent: H2
common solvent: pd/BaSO4 w Pbo OR pd/CaCO3 w pbo or quinaline
Dissolving Metal
-reduction reaction
regio: none
stereo: anti
adds: -H and -H
common reagents: Na,Li or K
Common solvent: NH3
Grignard
Ketone → tertiary alcohol
Aldehyde → secondary alcohol
Hydride nucleophile
Ketone → secondary alcohol
Aldehyde → primary alcohol
solvents for NaBH4: CH3OH, H3O+,
solvents for LiAlH4: H3O+, H2O
Organolithium
Ketone → tertiary alcohol
Aldehyde → secondary alcohol
Epoxide opening with weak nucleophiles
- nucleophile reacts to most substituted carbon
-examples: Br2 or H-Br
-H-Br forms alcohol
epoxide opening with strong/basic nucleophiles
-OCH3 reacts to least substituted carbon
-requires a workup (H3O+)
-example: NaOCH3/CH3OH
-forms alcohol
addition of weak nucleophiles to aldehydes
CH3OH/H2SO4
CH3OH/TSOH
additon of weak reversible nucleophiles
Specific acid cat
protonate
add
deprotonate
general mechanism
Add + Protonate
deprotonate
specific acid catalyst mechanism
protonate
add
deprotonate
protonate LG
assisted ionization (elimination)
deprotonate
weak base mechanism
3 step
add
deprotonate
LG leaves
5 step
add
LG leaves (elimination)
add
deprotonate
elimination of nucleophile
condensation reactions of hemis
condensation reaction of hemis mechanism via sn1
protonate to make good LG
LG leaves (assisted ionization)
addition of nucleophile to carbocation
deprotonate
condensation reaction of hemiaminal
mechanism of condensation of hemiaminal
hydrolysis reactions
ketal/acetal + H2O
mechanism of hydrolysis
protonate
LG leaves
Water adds
deprotonate water
Protonate LG
LG leaves
deprotonate
intramolecular substitution reactions
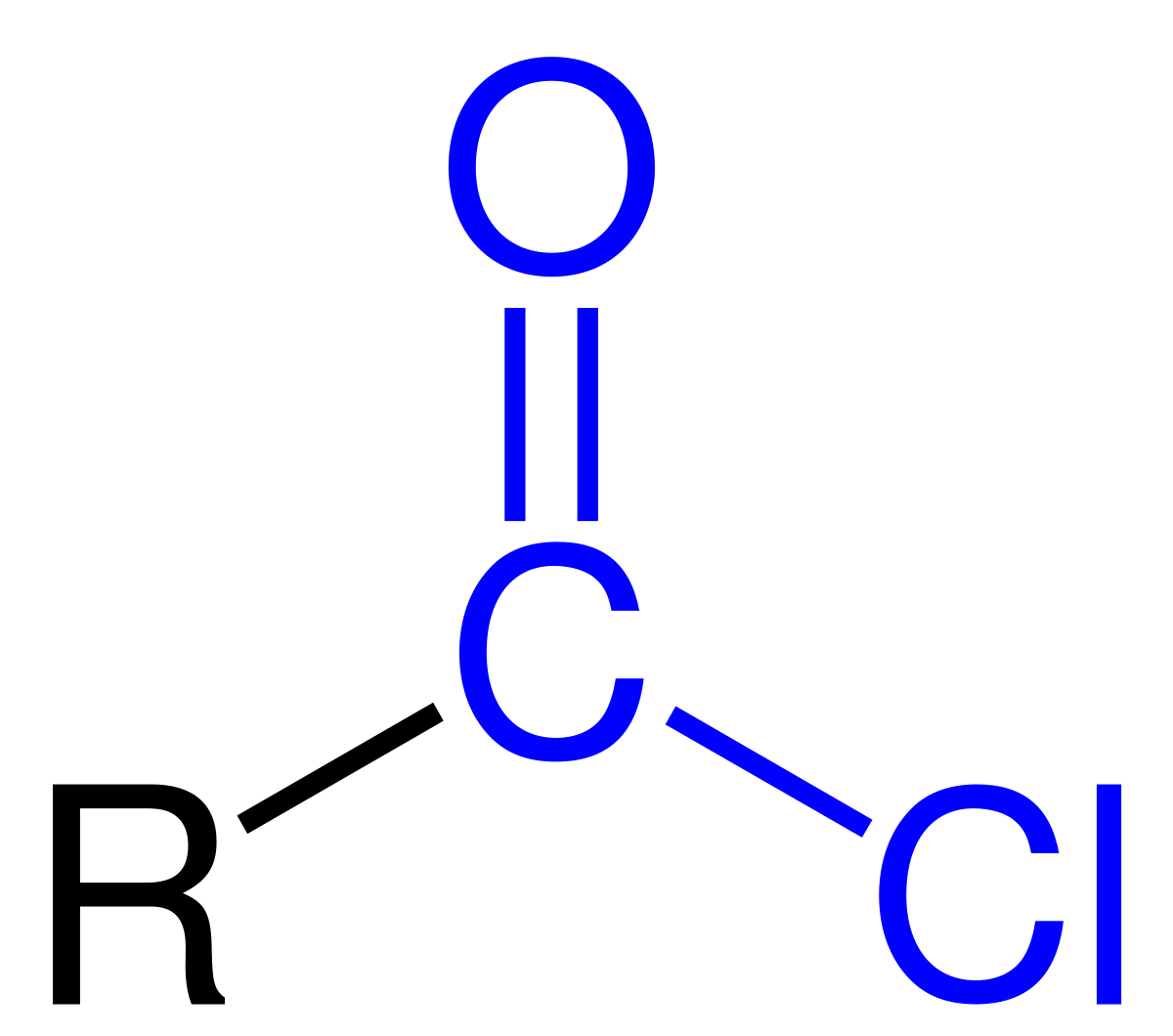
Acid Chloride
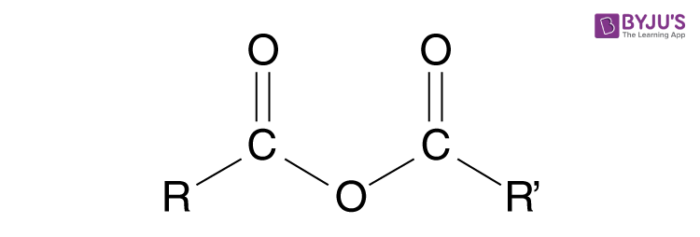
Anhydride
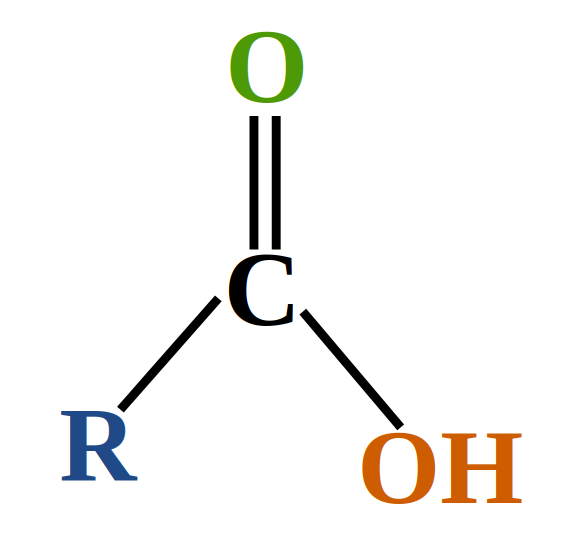
Carboxylic acid
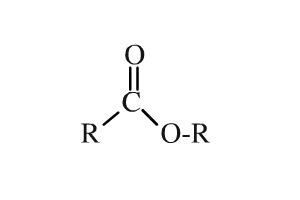
Ester
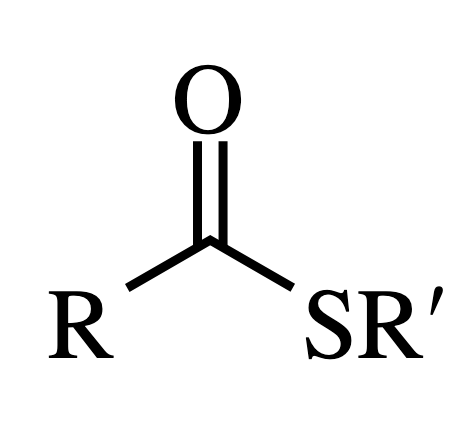
Thioester
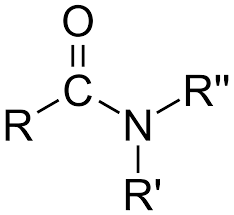
Amide
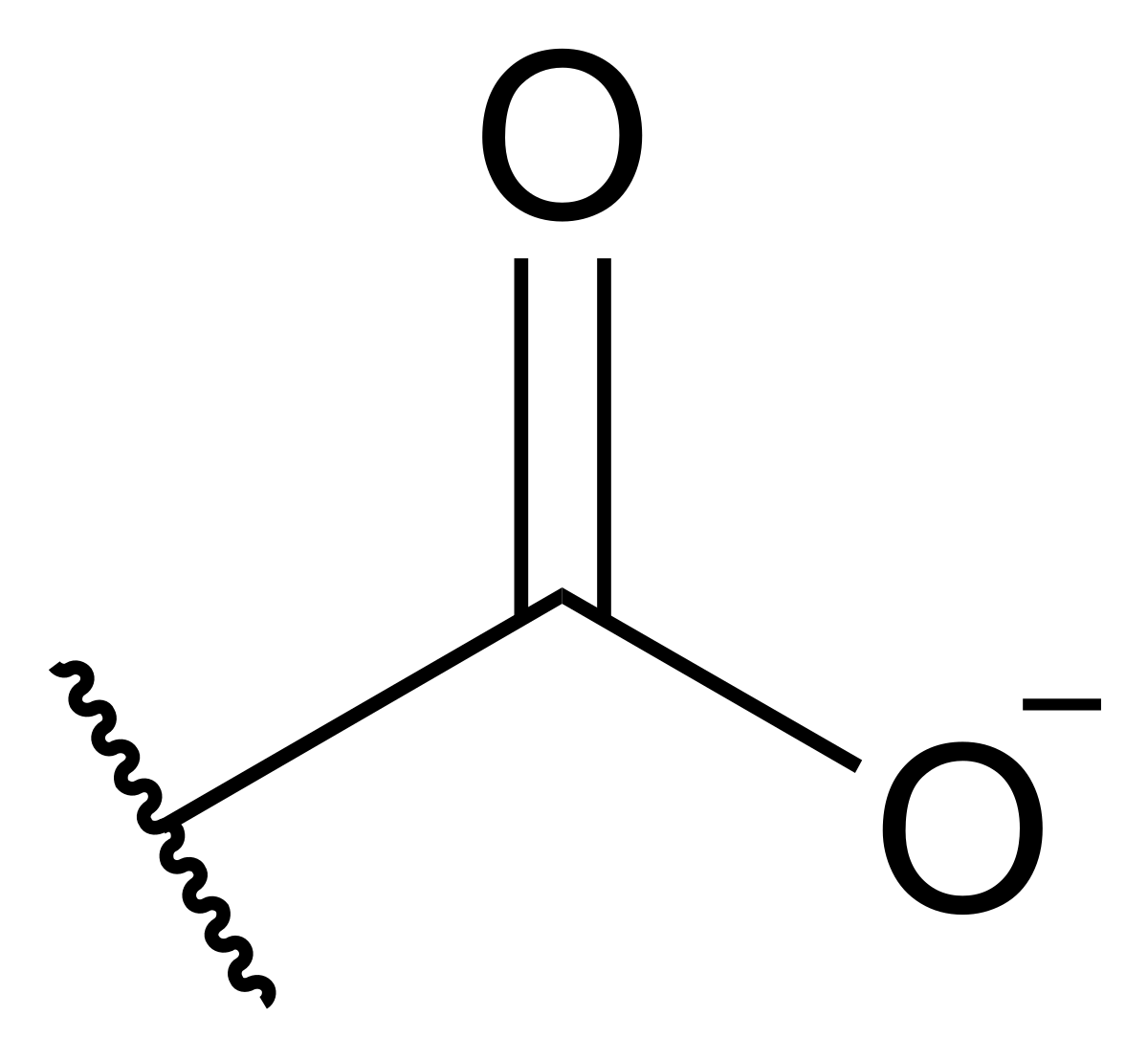
Carboxylate
Acylation
carbon in acyl group is the electrophile
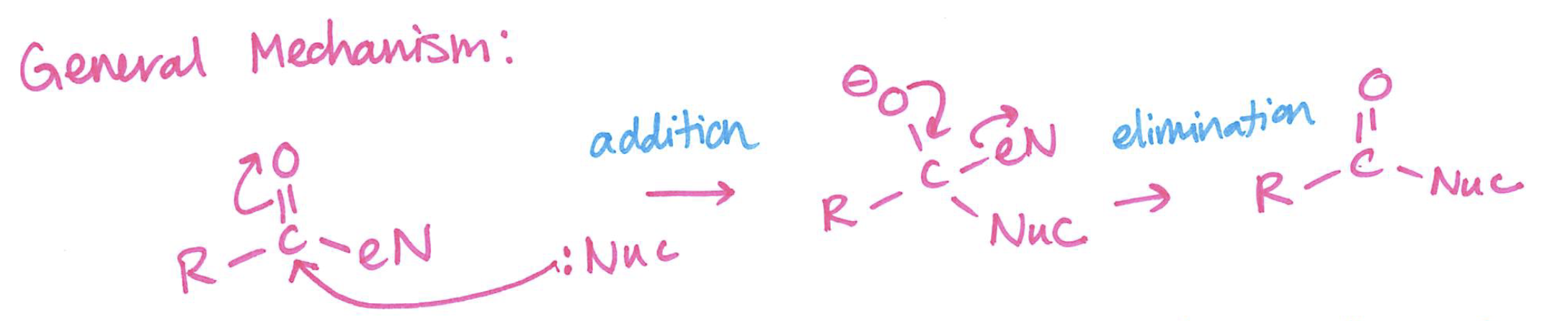
The one reaction
substitution at the sp2 carbon via addition and elimination
General mechanism for Acylation
addition
elimination
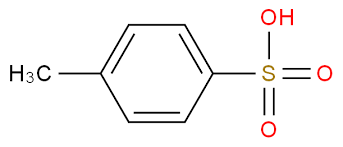
Tosic acid
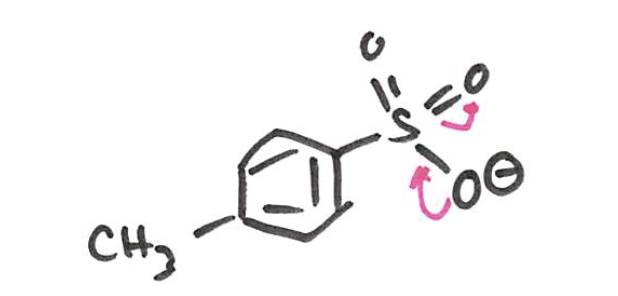
Tosylate
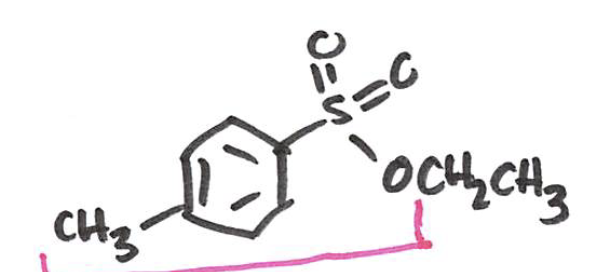
Sulfonate ester
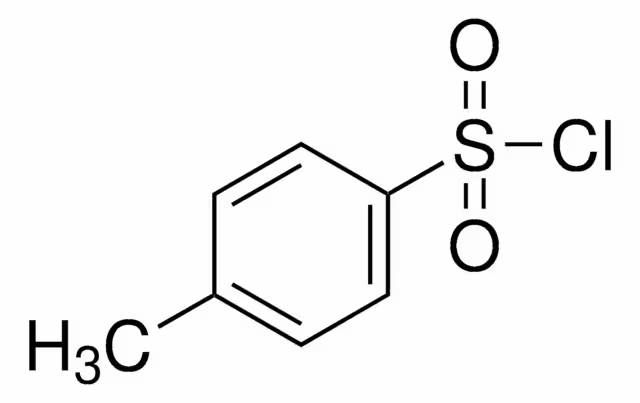
Tosyl chloride
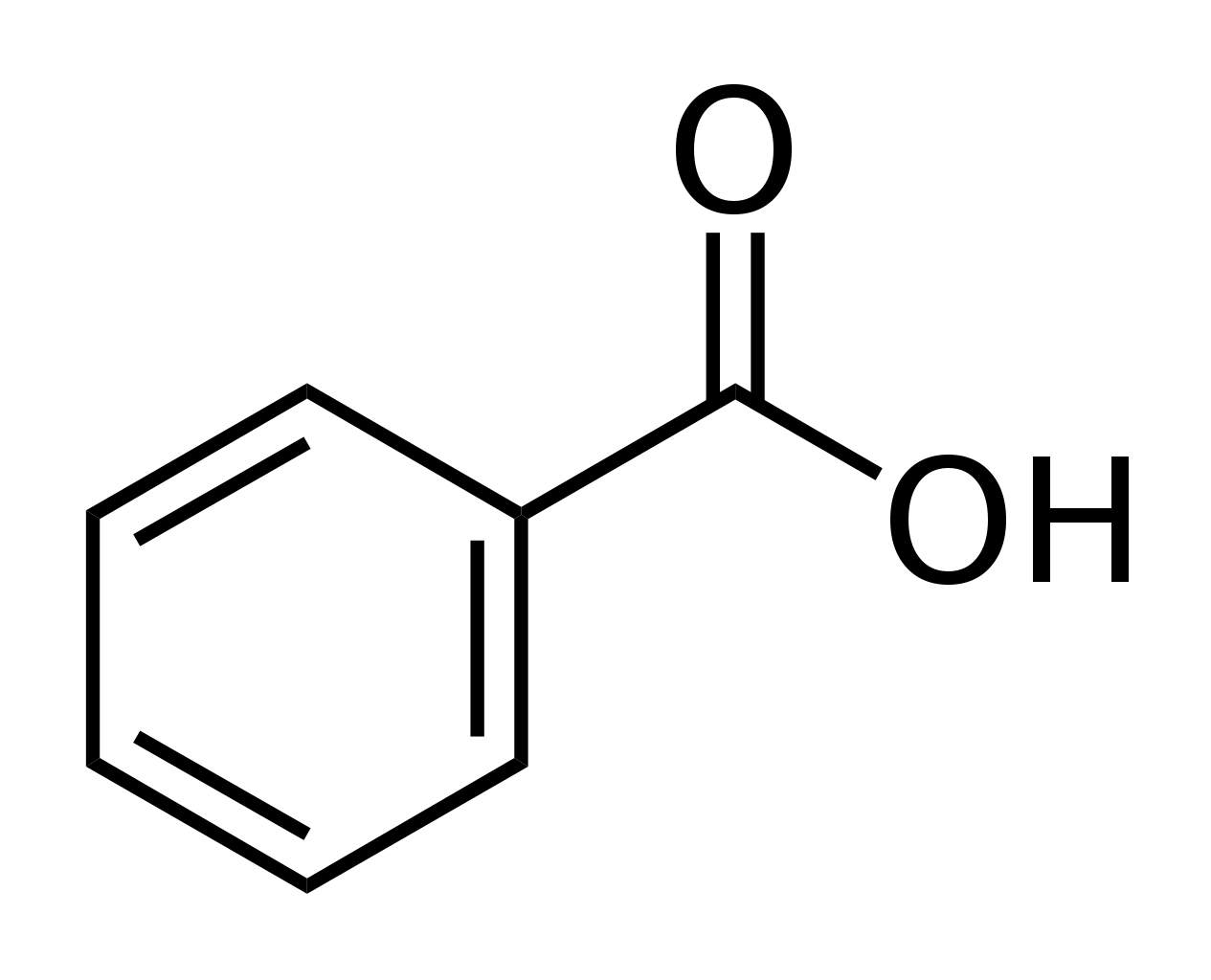
benzoic acid
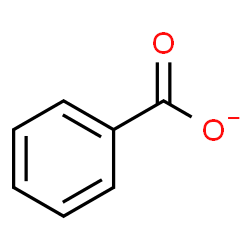
benzoate
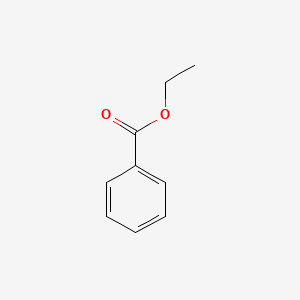
Ethyl benzoate
Strong base mechanism
2 steps
anions (-OCH3, -OH, -SCH3)
no strong acids present
solvents: CH3OH