Human Physiology
1/393
Earn XP
Description and Tags
Yr 1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
394 Terms
Total body water
60%
42L, 71mL/100g
Less in fat
Decreases with age
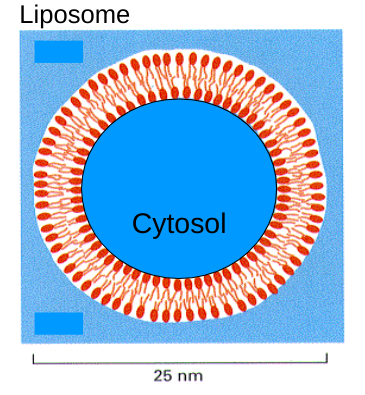
Liposome
If you put phospholipids into an aqueous solution, they would form a structure with the smallest surface area
Chemical disequilibrium and composition of body fluids
Extracellular: high Na+, high Cl-, low K+, low proteins (low in blood plasma, very low in interstitial fluid)
Intracellular: low Na+, low Cl-, high K+, high proteins (because of enzymes)
Different composition due to cell membrane’s permeability to molecules
Cell membrane permeability
Highly permeable to water
Larger size = lower permeability
Lipid soluble = increased permeability
Charge = cannot pass (most important factor)
Diffusion
Collisions cause random movement [high] to [low] down [gradient]
<3nm H2O soluble molecule can cross membrane this way
Can take place in open system or across partition that separates two systems
Filtration
High pressure through pores (holes in membrane)
E.g. kidney - phosphate ions pass through pores to be excreted in urine, proteins too big
Osmosis
Movement of ions across a membrane in response to a solute [gradient] (low to high solute gradient)
Cell = sealed environment
Osmotic pressure, P
Pressure which would prevent H2O moving. All compartments normally at osmotic equilibrium
Facilitated diffusion
[High] to [low]
down [gradient]
no energy needed
Carrier and channel proteins
Active transport
[Low] to [high]
Against [gradient]
ATP → ADP
ATPase releases energy
Endocytosis
Into cells
Proteins and very large molecules
Two types: pinocytosis and phagocytosis
Pinocytosis
Invaginated membrane pinches off pockets, e.g. fat uptake
Phagocytosis
Arms of cytoplasm encapsulate, e.g. immune response
Exocytosis
Out of cells
E.g. hormones and neurotransmitters
Two types of excitable cells
Muscle - smooth, skeletal and cardiac - contract
Nerve - neurones - conduct - send constant electrical signal over long distance
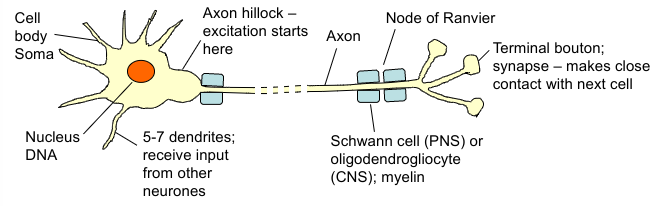
Neurones
Secretory - release chemicals called neurotransmitters at synapses
Cell body communicates with nerve terminals by electrical signals
Excitable cells - transmit electrochemical signals along their membranes by moving charged ions across the cell membrane = action potentials
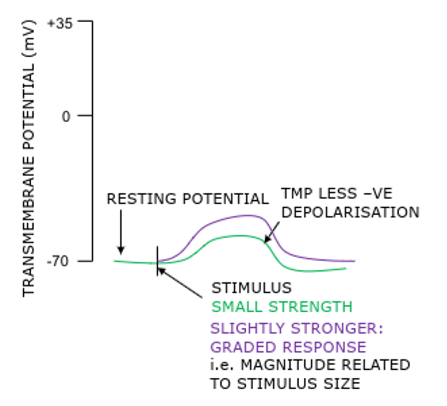
Transmembrane resting potential
Electrical gradient between extracellular and intracellular fluid; inside cell slightly negative
Electrical gradient = membrane potential, or Vm = voltage difference across cell membrane
A form of stored or ‘potential’ energy which can be used to open voltage-gated membrane channels and send electrical signals
-70mV inside cell
What creates transmembrane resting potential?
Diffusion of K+ (leak channels) out of cell
Channel always open
K+ crosses membrane down [gradient]
Sodium pump/Na+K+ATPase (active transport)
Moves 3Na+ out of cell and only 2K+ in
Keeps intracellular concentration of K+ high and Na+ low
Electrogenic pump - net loss of one positive charge from cytoplasm - helps maintain electrochemical gradient
Negative proteins inside cell
Voltage gated Na+ and K+ channels
Are normally closed in membrane at rest but are important in action potential generation
Opened by voltage change (depolarisation)
K+ channel slower to open and close
Both take time to reset
Membrane distribution for each ion is a balance
[gradient] and electrical gradient
E.g. Cl- leave down electrical gradient but enter down [gradient] - little transfer at rest
Na+ wants to enter down both gradients
Local, non-propagated response to transmembrane ion distribution changes
E.g. synaptic/graded or generator potential
Usually at dendrites and soma (EPSP, IPSP)
Loses strength through cytoplasm
Propagated disturbance of transmembrane ions
Action potential
At axon
All-or-none response - if sufficient stimulus get constant amplitude response
Does not diminish in strength as travels through neurone
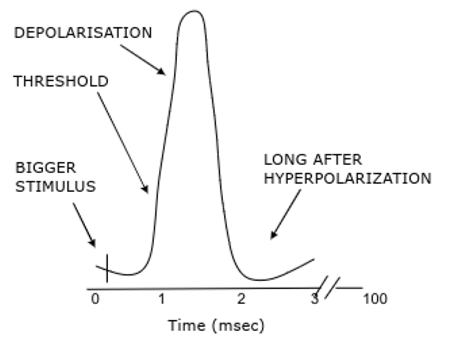
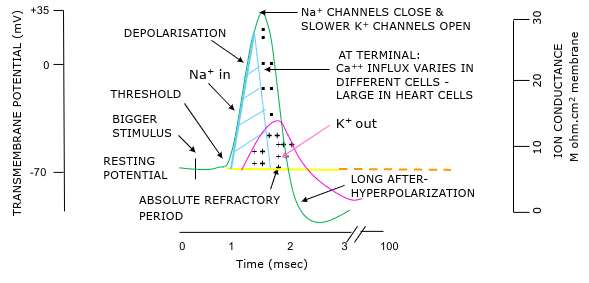
Action potentials
Transient reversal in potential
All or nothing response - if sufficient response get constant amplitude response
Larger stimulus → increased frequency of action potentials
Absolute refractory period = cell cannot fire = no more AP - ensures one way travel of AP
Relative refractory period = only larger-than-normal stimulus can initiate new AP
Local anaesthetics block Na+ channels to stop action potentials
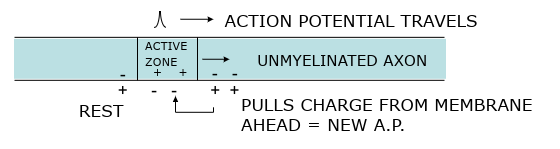
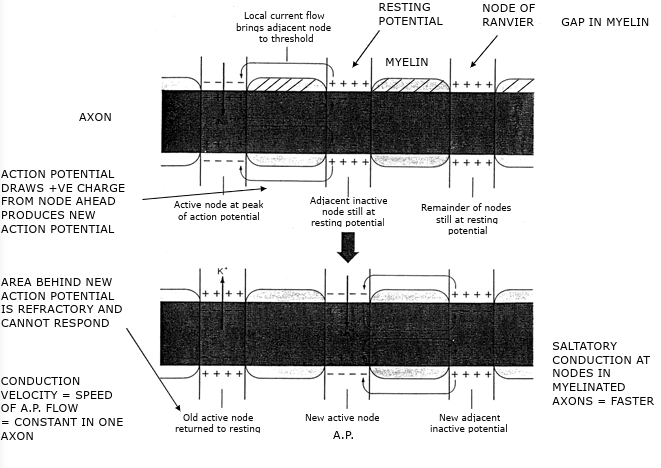
Action potential movement
Starts at axon hillock
Travels in one direction down axon
Orthodromic conduction
To nerve endings = synapse
Only pass message in one direction
Each section of the axon experiences a different phase of the action potential
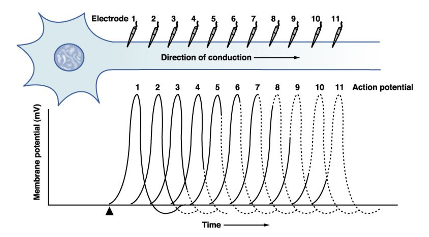
Myelinated axon
Postsynaptic potentials
Local graded change in transmembrane potential
Not propagated and quickly decay in intensity and distance
No refractory period
Therefore can summate
Change postsynaptic cell’s excitability making it more or less likely to fire an action potential
Excitatory Postsynaptic Potential (EPSP)
ACh neurotransmitter
At NMJ 2 ACh bind and (nicotinic acetylcholine) receptor changes shape
Opens Na+ channel - depolarisation
Small, graded, local, short-lasting depolarisation of postsynaptic membrane
If threshold for action potential is reached, muscle contracts/AP generated in postsynaptic neurone
More likely to fire AP
Inhibitory postsynaptic membrane (IPSP)
GABA - inhibitory neurotransmitter
Chloride enters
Cell made more negative
Inside local hyperpolarisation
Transmembrane potential further from threshold
Less likely to fire AP
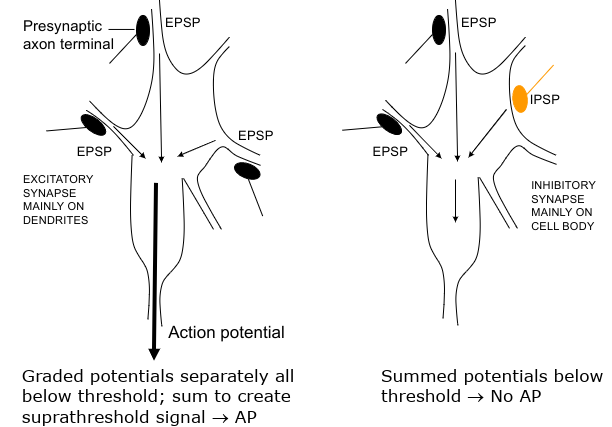
Convergence
Summation of IPSP and EPSP
Excitatory synapse mainly on dendrites
Inhibitory synapses tend to be on cell body
Synapses closest to axon hillock have greatest effect on action potential and firing
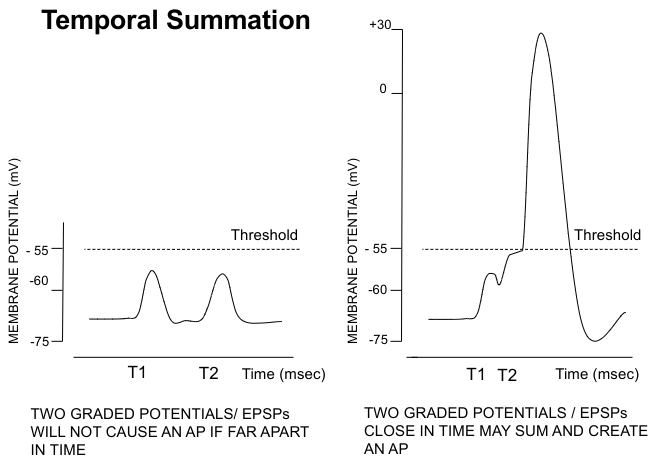
Spatial summation and temporal summation
Spatial summation
2 EPSPs on adjacent membrane add together
Temporal summation
2 EPSPs close in time can add, IPSP and EPSP can cancel out
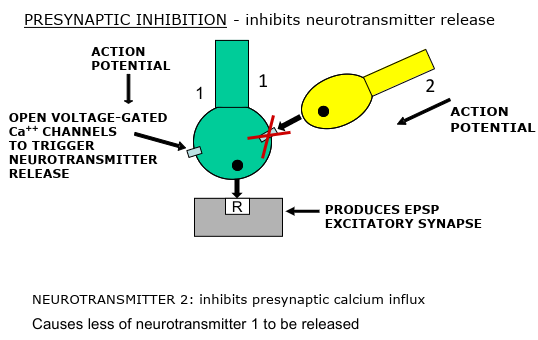
Modulation of synaptic activity at the axon terminal
Amino acid neurotransmitters
Glycine
GABA
Classical neurotransmitters
Noradrenaline
Acetylcholine
Peptide neurotransmitters
TRH
Substance P
Other neurotransmitters (not peptides, amino acids, or classical)
ATP
Nitric oxide
Autonomic nervous system
‘Visceral nervous system’ - control over internal organs
Subconscious control - heart rate, pupil diameter, blood vessel contractility, hormonal secretions, gastrointestinal motility
Homeostasis
Afferent
Goes to CNS
Efferent
Goes away from CNS
Organisation of autonomic nervous system
Axons have:
A two-neurone chain
Preganglionic (first) neurone extends to ganglion
Postganglionic (second) neurone extends from ganglion to effector organ
Ganglia = cell bodies of many peripheral autonomic neurones occurs in clusters and form swellings on nerve trunks
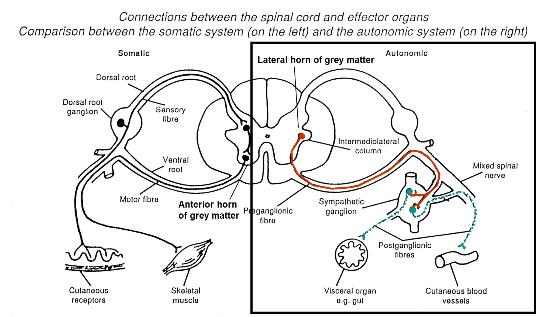
Motor (efferent) pathways of ANS
Axons that form synapses with ganglion cells = preganglionic autonomic fibres
Axons innervating effector cells = postganglionic autonomic fibres
Postganglionic neurone has many varicosities from which a neurotransmitter is released
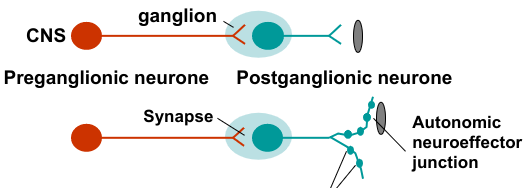
Neurotransmitters of the autonomic nervous system:
Preganglionic nerves
Postganglionic nerves
Pre and postganglionic nerves
Acetylcholine - cholinergic transmission
ACh and Noradrenaline (noradrenergic transmission)
Non adrenergic non cholinergic neurotransmission (NANC) and co-transmitters (neuromodulators)
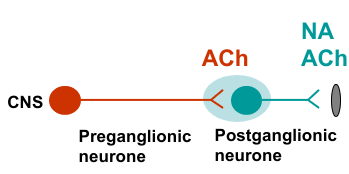
Sympathetic vs parasympathetic
Function separately
Have opposing effects in some states (e.g. heart rate, GI tract) but not others (e.g. salivary gland secretion, blood vessels)
Sympathetic activity increases in stress (aroused state, fight or flight, pupils dilated, hair erected, increased blood sugar, increased heart rate, increased blood flow through muscle, blood diverted from GI tract)
Parasympathetic activity predominates during satiation and repose - rest and digest
Both systems exert continuous physiological control under normal conditions, at neither extremes - cooperate
Influences by sensory information via control centres in the brain
Parasympathetic nervous system pre and postganglionic fibres
Preganglionic axons emerge from cranial and sacral regions of CNS
Preganglionic axons form synapses in ganglia near to/adjacent to/within effector tissues
Sacral nerves - form pelvic plexuses containing scattered ganglia and some ganglia within tissues - pelvic and abdominal viscera (bladder, rectum, genitalia etc.)
Parasympathetic preganglionic fibres are long, postganglionic fibres are short

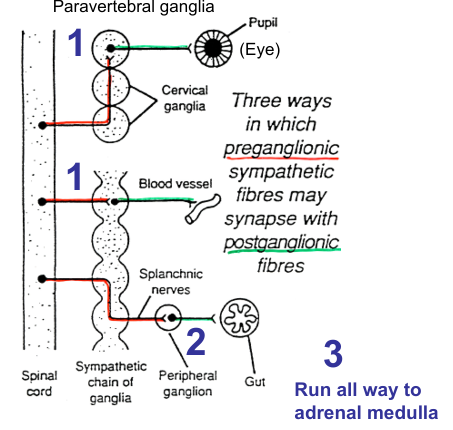
Sympathetic nervous system pre and postganglionic fibres
Preganglionic sympathetic axons entering chains terminal in:
Paravertebral sympathetic chains
Both pre and post ganglionic axons may run for some distance up or down the sympathetic chain before forming synapse/emerging
Prevertebral ganglia/plexuses
In the abdominal cavity: 3 main - coeliac ganglion, superior mesenteric ganglion, hypogastric (or inferior mesenteric) ganglion
Adrenal medulla
Some preganglionic fibres emerging from 10th and 11th thoracic segments run in greater splanchnic nerve and terminate on chromaffin cells in the medullae of adrenal glands. Chromaffin cells analogous to sympathetic ganglia cells
In general, sympathetic preganglionic fibres are short, postganglionic fibres long
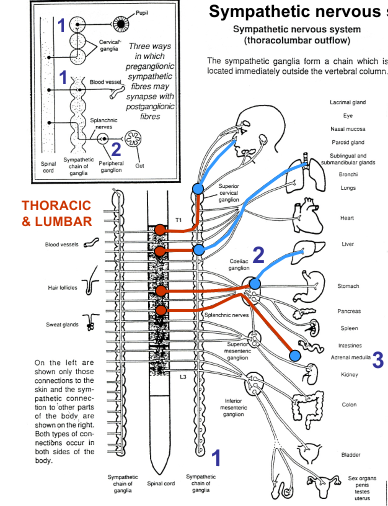
Different ways in which preganglionic sympathetic fibres may synapse with postganglionic fibres
Parasympathetic nervous system
AKA craniosacral division
Keeps body energy use low
Predominates in individuals in relaxed states
Blood pressure, heart rate, respiratory rates low
GI activity high
Rest and digest
Inhibitory effect on many tissues and organs
Cranial outflow of parasympathetic nervous system
Oculomotor (III) - smooth muscles of eyes
Facial (VII) - facial glands (lacrimal, salivary)
Glossopharyngeal (IX) - salivary glands
Vagus (X) - postganglionic fibres usually in target organ: heart, lungs, bronchi, stomach, oesophagus, liver, small intestine, pancreas, kidneys, proximal part of large intestine
Sacral outflow of parasympathetic nervous system
Nerves from pelvic plexuses containing scattered ganglia. Some ganglia are within tissue
Nerve innervate distal part of large intestine, bladder, ureters, reproductive organs
Parasympathetic neuroeffector pathway
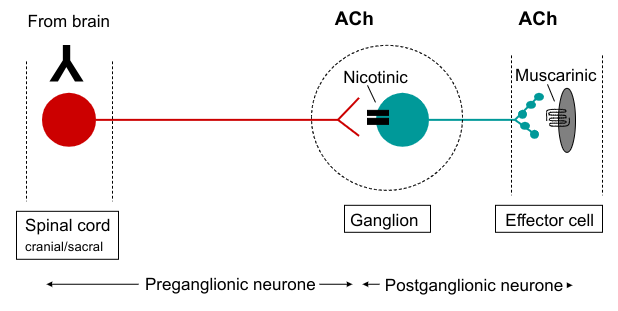
Neurotransmitter synthesis, action and degradation at cholinergic synapses (parasympathetic)
ACh acts postjunctionally on nicotinic receptors at ganglionic synapses
ACh acts postjunctionally on muscarinic receptors at effector cell, e.g. heart and smooth muscle
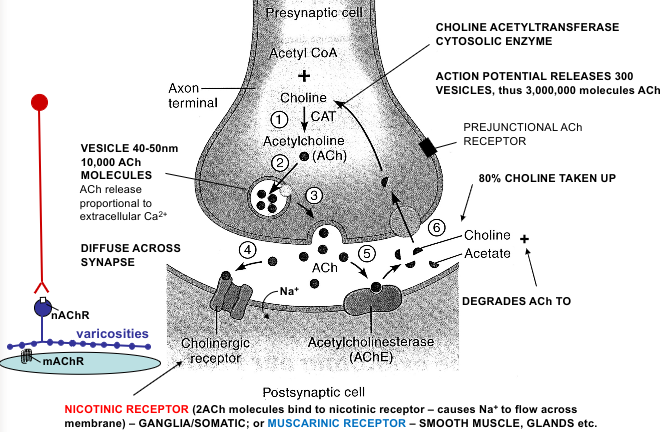
ACh receptors
Nicotinic - at ganglia (and brain, neuromuscular junction). Ionotropic
Muscarinic - at autonomic target tissues (and brain). Metabotropic (M1-M3)
M1
Neuronal/gut (gastric acid secretion increased)
M2
Cardiac/presynaptic (heart rate/force decreased)
M3
Glandular (secretion increased), smooth muscle (contraction)
Parasympathetic drugs
Parasympathomimetic - cholinomimetic agonists (pilocarpine, muscarine, nicotine) - increased PNS
Parasympatholytic - muscarinic antagonists (atropine) - decreased PNS
Cholinesterase inhibitors (ACHEI) - increased PNS
Ganglionic blocking drugs - nicotinic antagonist - blocks both sympathetic and parasympathetic ganglia - decreased PNS and SNS
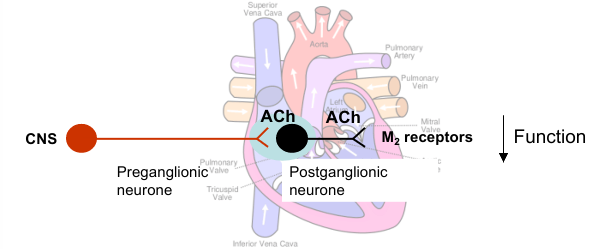
Parasympathetic control of heart
Postganglionic nerves release ACh which acts at M2 muscarinic receptors to decrease heart function
Sinoatrial node (pacemaker) - decreases heart rate (bradycardia)
Atrial muscle - decreases contractility (force and duration of contraction)
Atrioventricular node (conducts electrical impulses) - decreases rate of conduction
(Cardiac arrest - atropine (muscarinic antagonist) used for resuscitation)
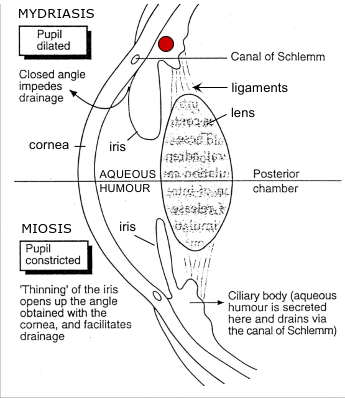
Parasympathetic control of pupil diameter
Circular muscle/constrictor pupillae = smooth muscle arranged concentrically
Parasympathetic nerve activation → ACh → M3 muscarinic receptors → circular muscle contraction → pupil constriction/miosis
Inhibition of contraction → muscarinic antagonist (e.g. atropine) → blocks circular muscle contraction → passive dilation → myrdriasis
Parasympathetic control of focussing
Ciliary muscle relaxed
Suspensory ligaments taut
Lens flattened
Focus for distant vision
Ciliary muscle contracts and moves in due to ACh action at M3 receptors
Suspensory ligaments relax
Lens gets fatter
Focus for near vision/accommodation
Cycloplegia - paralysis of ciliary muscle → loss of accommodation
Mydriatic drugs (parasympathetic)
Muscarinic antagonist - passive dilation/mydriasis (loss of drive to constrictor pupillae) and cycloplegia (loss of accommodation to ciliary muscles)
Antiglaucoma and miotic drugs (parasympathetic)
Muscarinic agonist - pressure in eye builds up due to production of fluid (aqueous humour) and lack of drainage through canal of Schlemm. Dilated pupil - iris occludes canal. Muscarinic agonist constricts pupil/miosis → moves away from canal of Schlemm to increase outflow and decrease intraocular pressure
Anticholinesterases - enhancement of parasympathetic stimulation: miosis and accommodation (due to contraction of ciliary muscle)
Parasympathetic cotransmission
ACh and VIP (vasoactive intestinal polypeptide)
Low frequency stimulation - ACh released
High frequency stimulation - ACh and VIP released
Other cotransmitters: ATP and NO
Most sympathetic neuroeffector pathways
ACh and NA
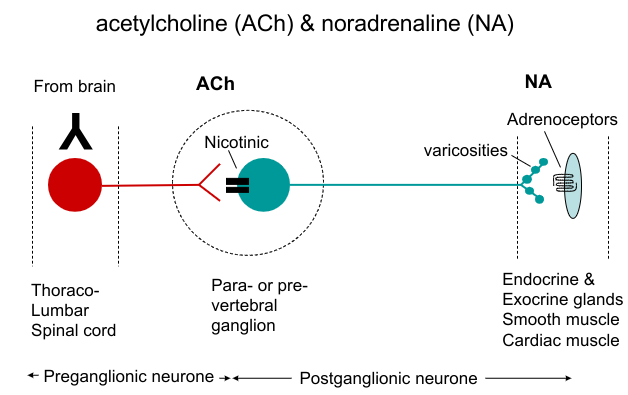
Other sympathetic neuroeffector pathways
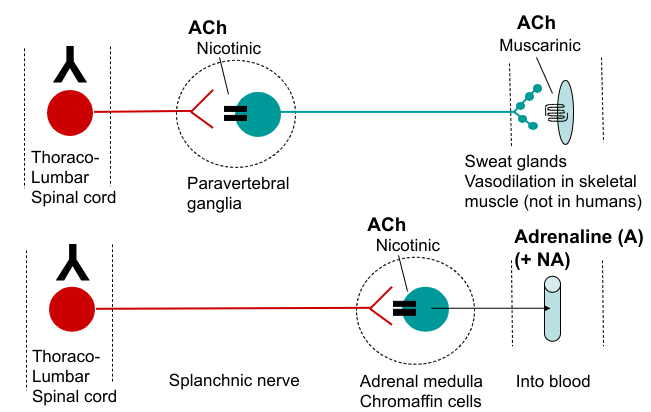
Autonomic neuroeffector junction
Controls autoinhibition (fine tuning amount of NA released)
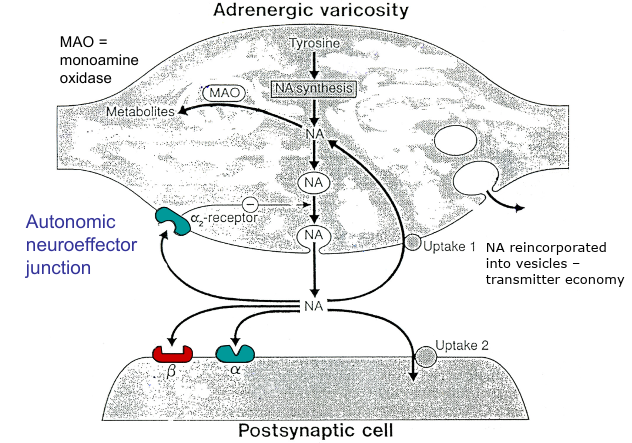
Adrenoceptors
Metabotropic
On smooth muscle, endocrine and exocrine glands, cardiac muscle
Alpha 1 and 2 and beta 1, 2 and 3 are all postjunctional
Alpha 2 is the only prejunctional
Agonists: noradrenaline, adrenaline, isoprenaline
Agonist potencies for alpha adrenoceptors: NA > A > ISO
Agonist potencies for beta adrenoceptors: ISO > A > NA
Alpha 1 agonist - decongestant, glaucoma, mydriasis
Alpha 1 antagonist - antihyperintensive
Beta 1 agonist - enhance cardiac muscle contraction
Beta 2 agonist - bronchodilator (Salbutamol)
Beta antagonists - beta blockers - cardiac arrhythmias, angina, essential hypertension, cardioprotection after myocardial infarction
Diverse drug action at adrenergic varicosity (sympathetic nervous system)
Postjunctional actions
alpha or beta receptor agonist - increase SNS
alpha or beta receptor antagonist - decrease SNS
Prejunctional actions
alpha 2 receptor agonist - decrease SNS
alpha 2 receptor antagonist - increase SNS
NA uptake inhibitor - increase SNS
MAO inhibitor - increase SNS
Stimulate release - increase SNS
Impair/cause depletion release - decrease SNS
Impair synthesis - decrease SNS
False neurotransmitter - decrease SNS
Sympathetic control of pupil diameter
Radial muscle/dilator pupillae = smooth muscle arranged radially
Sympathetic nerve action → NA → alpha adrenoceptors on radial muscle → radial muscle contraction → pupil dilation/mydriasis
Mydriatic drugs (sympathetic)
Eye inspection and surgery
Sympathomimetic
Mydriasis due to contraction of dilator pupillae
Antiglaucoma drugs (sympathetic)
Sympathomimetic
Glaucoma - pressure in eye due to build up of aqueous humour and lack of drainage through canal of Schlemm
Vasoconstriction caused by sympathomimetic impairs secretion of aqueous humour from ciliary body and facilitates reabsorption from canal of Schlemm thus easing pressure
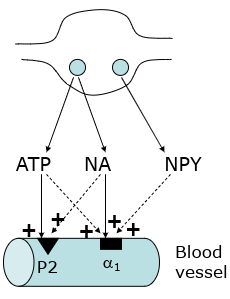
Sympathetic cotransmission
NA + NPY (neuropeptide Y) + ATP
Act together to cause vasoconstriction
NA works on alpha 1 receptors, ATP works on P2 receptors
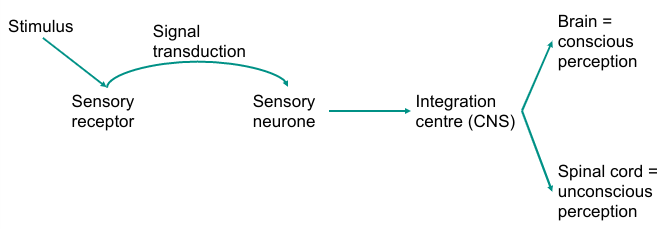
Peripheral nervous system
Relay sensory information from periphery and internal environment into CNS via afferent pathways
Relay motor output from CNS to skeletal and smooth muscles via efferent pathways
Part of somatic nervous system
Voluntary responses
Information processed by somatic senses
Touch, temperature, pain, proprioception (awareness of body’s location and movements)
Information processed by special senses
Smell (olfaction), taste, hearing, balance, vision
Information processed by visceral senses
Blood pressure, distension of GI tract, internal body temperature, blood glucose control, internal pH
Common sensory pathway
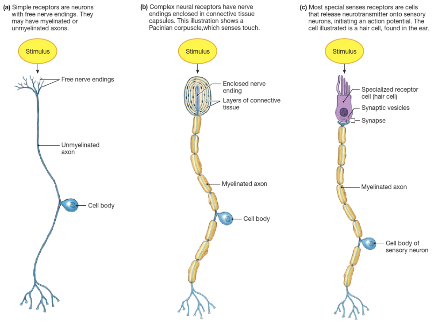
Sensory receptors
Somatic sensory receptors = modified or free nerve endings of sensory neurones
Special sense receptors = receptor cells that synapse with sensory neurone
Located throughout body
Activated by specific stimuli
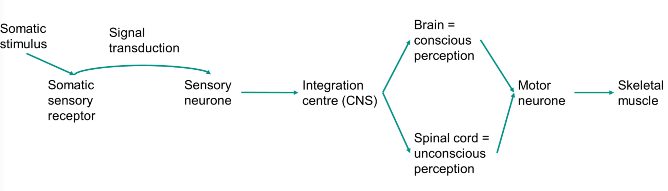
Somatic nervous system pathway
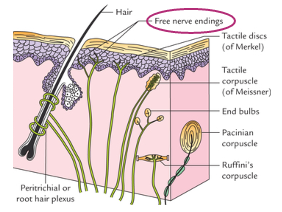
Mechanoreceptors
Location: mostly skin (also visceral organs)
Stimuli = physical distortion (touch)
Subtypes:
Meissner’s corpuscles - glabrous, low threshold touch
Merkel’s disk - glabrous, low threshold static touch
Ruffini’s corpuscles - glabrous and hairy, high threshold stretch
Pacinian corpuscles - largest, deepest vibration
Free nerve endings: glabrous and hairy, very high touch threshold
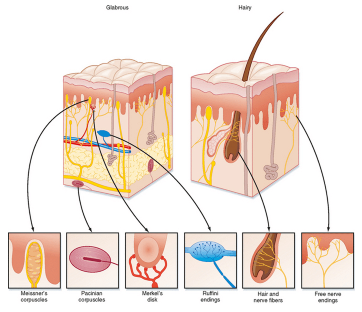
Mechanoreceptor adaptation
Meissner’s and Pacinian corpuscles - action potential firing at the onset and offset of the stimulus only - rapidly adapting
Merkel’s disks and Ruffini’s endings - action potential firing throughout the presence of the stimulus = slowly adapting
Thermoreceptors
Location: mostly skin
Stimuli: temperature
Free nerve ending ion channels: TRPV1 - hot, TRPM8 - cold
Nociceptors
Location: mostly skin
Stimuli: stimuli that have the potential to cause tissue damage
Free nerve endings:
Mechanical nociceptors
Thermal nociceptors
Chemical nociceptors
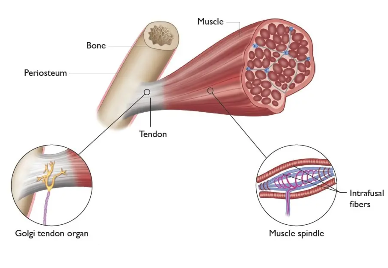
Proprioceptors
Location: muscles, tendons, ligaments, joints
Stimuli: muscle tension
Subtypes:
Muscle spindles (aka stretch receptors) - muscle length (stretch)
Golgi tendon organ - muscle tension (force of contraction)
In joints - angle, direction and velocity of movement in a joint
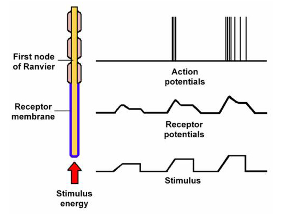
Sensory transduction
Converting stimulus energy into electrical signals
Activation of receptor causes the movement of ions = change in membrane potential
Receptor = receptor potential
Sensory neurone = action potential
Receptive fields
Area in which the specific stimulus will activate the sensory receptor
High density of smaller fields = higher spatial resolution of the stimulus
Fingers and lips = highly discriminative
Back, leg and arm = less discriminative
Primary afferent neurones
Axons of neurones bringing in information from the somatic sensory receptors to the spinal cord
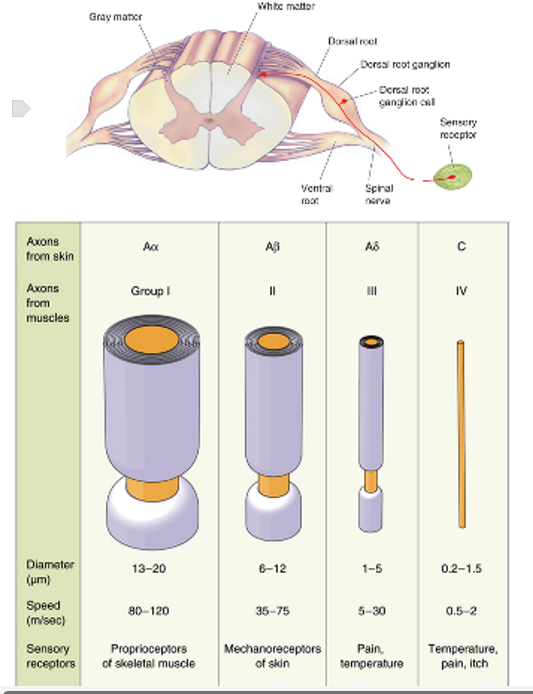
Primary afferent neurones have their cell body in the dorsal root ganglia and their axons project into the dorsal horn of the spinal cord through the dorsal roots
I, II, III - myelinated
IV - unmyelinated
Ascending tracts
Dorsal column-medial lemniscal pathway - touch and proprioception (from large dorsal root axons, up dorsal column through nuclei, through thalamus, to primary somatosensory cortex)
Spinothalamic pathway - temperature and pain (from small dorsal root axons, through medulla, through thalamus, to primary somatosensory cortex)
Somatosensory cortex
Lower parts of body get higher parts of cortex
Different parts of body get bigger parts of cortex depending on sensory innervation - hands and lips get bigger parts than back, for example
Taste
Tastants interact with particular taste receptor cell
Interaction leads to release of serotonin - able to bind to primary gustatory neurones
Binding of a neurotransmitter leads to action potentials which travel along axons
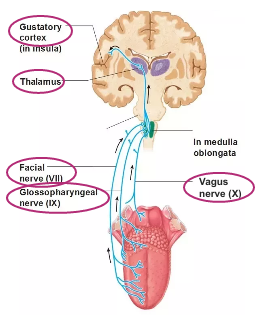
These axons of primary gustatory neurones make up 3 different types of cranial nerves - facial, glassopharyngeal and spinal nerves
Information transmitted along axons of these nerves straight to brainstem
No information going to spinal cord with special senses
Cranial nerves extend from brain and brainstem
Information is synapsed with second order neurone, which transmits info up to thalamus, key sensory relay centre, where it synapses with the third neurone that terminates win gustatory cortex in brain that allows us to interpret and process info about taste
Receptor cells all respond to slightly different tastes - how we’re able to differentiate
Hearing
Ability to detect auditory information in the form of sound waves
Enter inner ear via ear canal
Detected by particular sensory receptor cell - hair cells
Hair cells express cilia on top
Sound waves cause cilia to bend
This causes opening of ion channels
This causes a change in membrane potential leading to release of glutamate
Glutamate combines to primary auditory neurones, triggering action potential
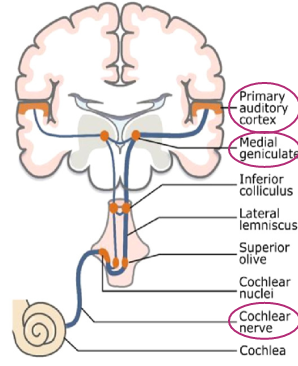
Axons of primary auditory neurones make up 8th cranial nerve, the cochlear nerve
Info transmitted via brain stem to thalamus, to auditory cortex found in temporal lobes
Vision
Receiving visual info in the form of photons of light
Info transmitted through pupil of eye and hits retina in back
Hits particular sensory receptor cells in retina - photoreceptors - able to detect this particular light
Photoreceptors that express a specific protein are activated by specific wavelengths of light - causes change in membrane potential, getting a movement of ions
Allows change in neurotransmitter passed on to next cell
Photoreceptors not directly innervated by primary sensory neurones
Info passed on through more neurones before it reaches retinal ganglion cells
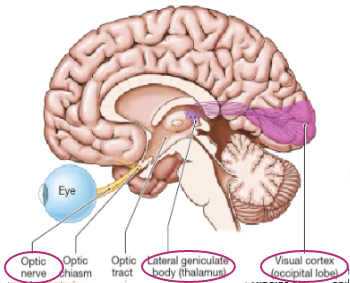
Axons of retinal ganglion cells make up second cranial nerve, optic nerve
Info passed along via optic nerve via optic tract, to thalamus then to third order neurone which terminates in visual cortex in occipital lobe
Olfaction
Odorants dissolve in olfactory epithelium that lines the naval cavity
Different odorants detected by receptors expressed on olfactory sensory neurones themselves
These are essentially free nerve endings - not receptor cells
Free nerve endings express particular receptors so that different odorants can be detected and causes a change in membrane potential
Axons of olfactory sensory neurones form part of first cranial nerve, the olfactory nerve, which forms part of olfactory bulb
Sensory info goes from nose via olfactory tract
Bypasses thalamus and is instead projected to piriform cortex or olfactory cortex for processing
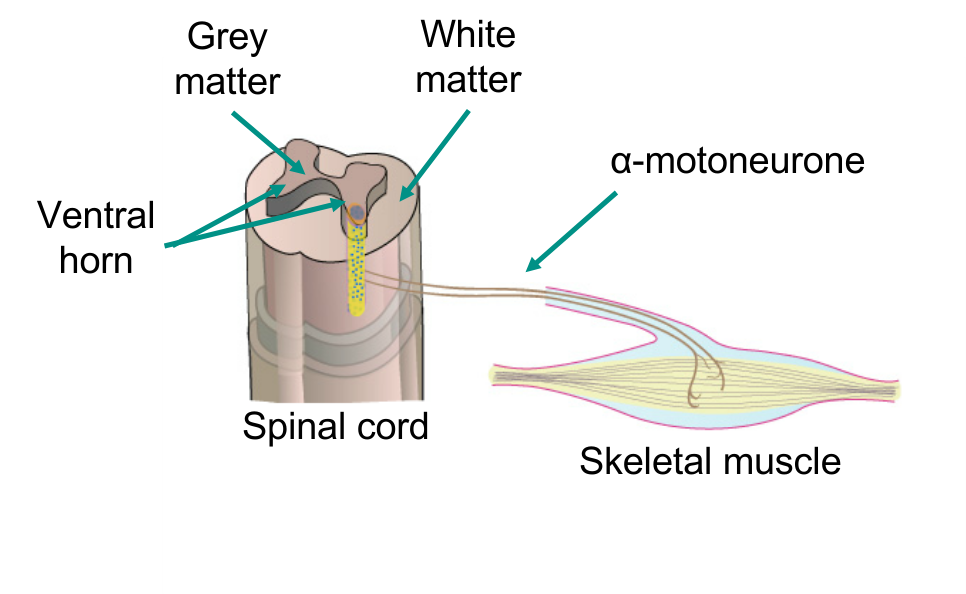
Alpha motoneurones
Motor neurones of the spinal cord
Cell bodies in ventral horn
Axons project out to skeletal muscle
Synapse with muscle fibres - neuromuscular junction
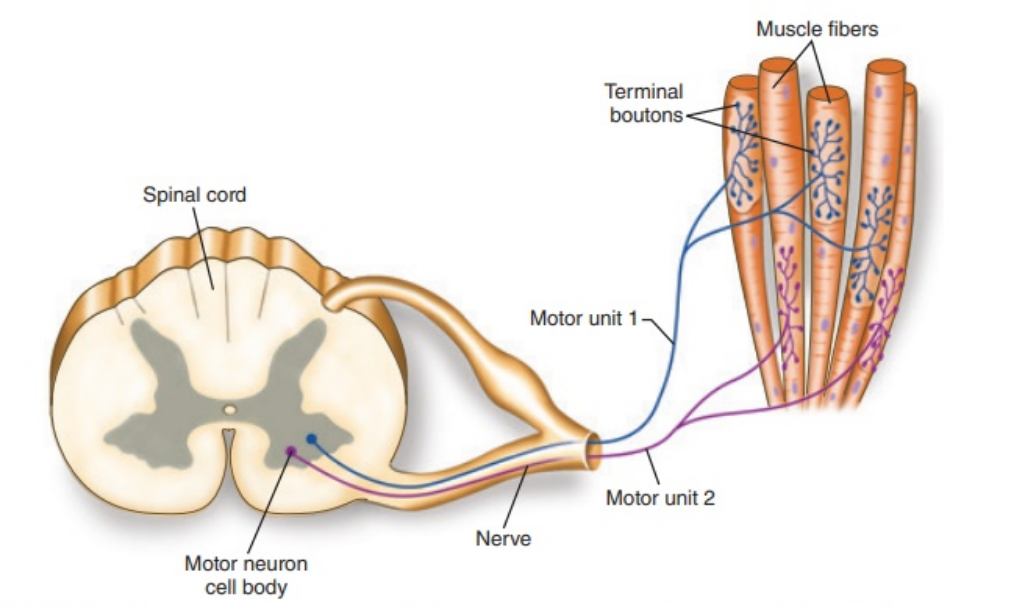
Motor unit
Skeletal muscles made up of bundles of muscle fibres
Motor unit = single alpha-motoneurone and all the muscle fibres it innervates
Each alpha-motoneurone can synapse with multiple muscle fibres
Each muscle fibre receives input from a single alpha motoneurone - focal innervation
Safety feature - nerve terminals always release 8-10x more ACh than necessary so we always get muscle contraction with a single action potential
Neuromuscular junction
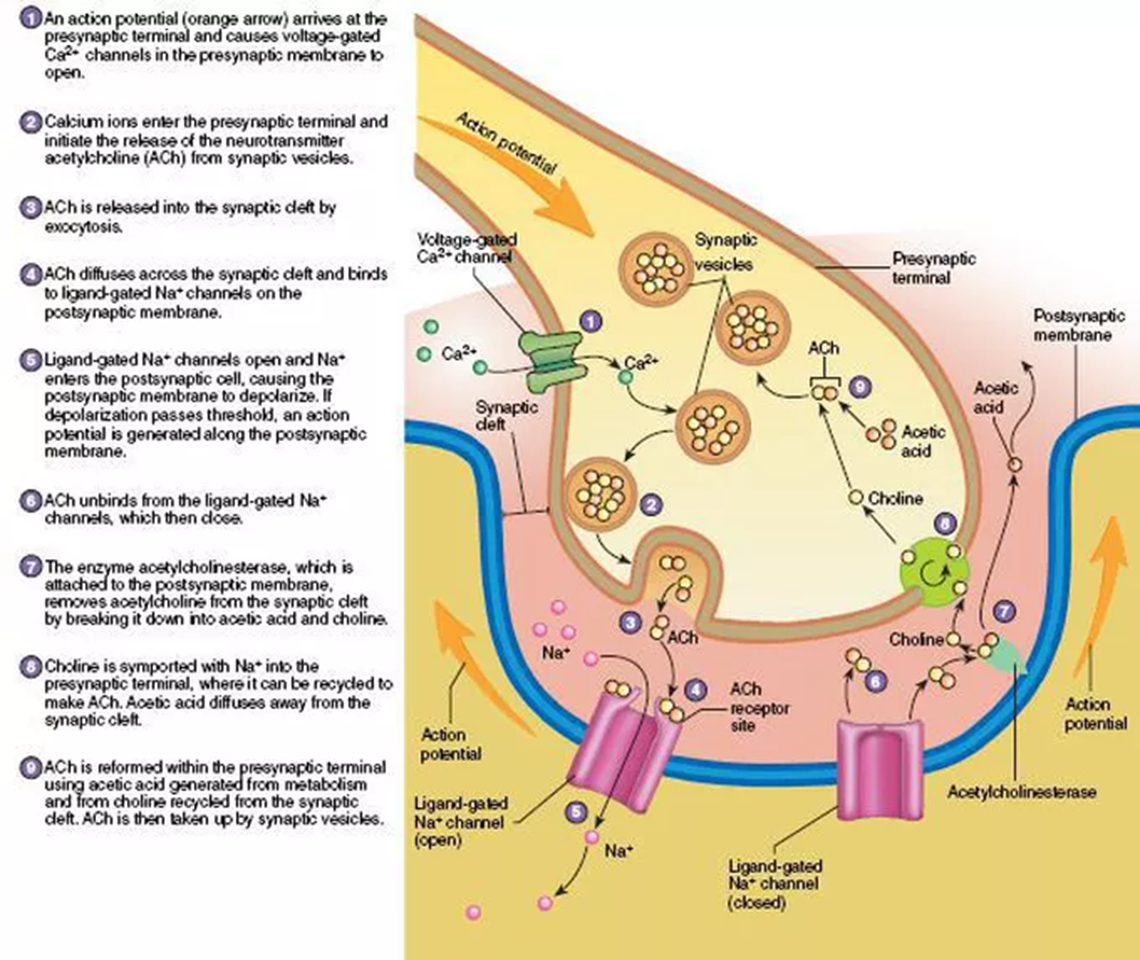
Tubocurarine
Nicotinic receptor antagonist
Blocks receptors, prevents ACh binding - numbs muscle
Smooth contraction
Each alpha-motoneurone innervates muscle fibres that are spread throughout the muscle
Alpha-motoneurones fire asynchronously
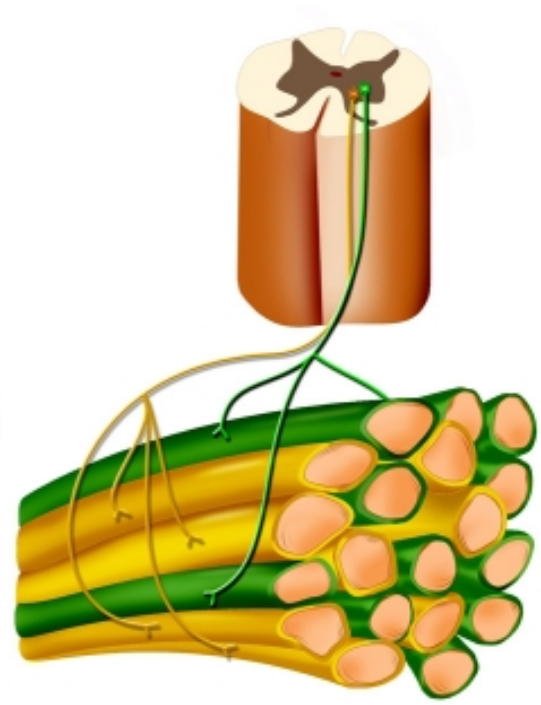
Contractile precision
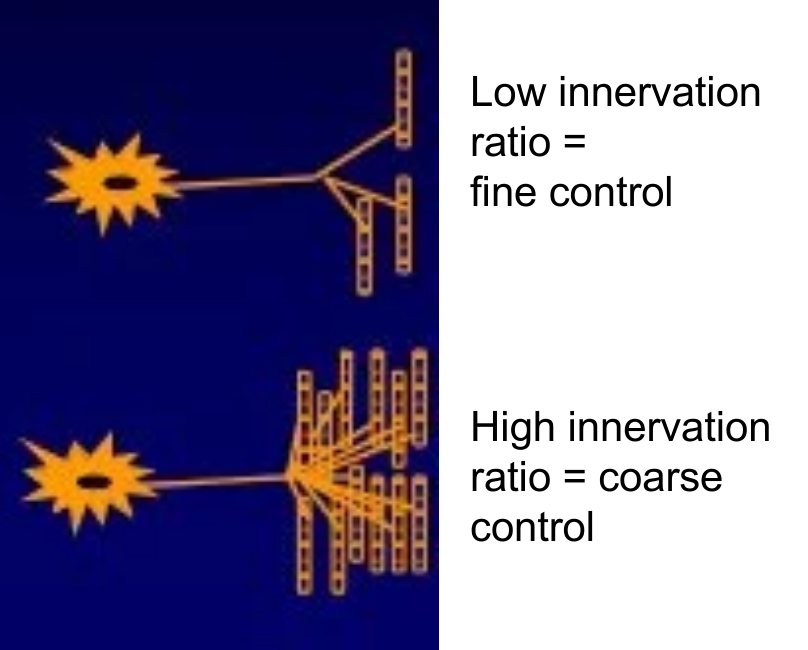
Innervation ratio = number of muscle fibres innervated by each individual alpha-motoneurone
Innervation ratio inversely correlated with contractile precision
Fingers = very dextrous, 5-15 fibres per alpha-motoneurone
Abdominal muscles - course movements - 200-1500 fibres per motoneurone
Type I/slow twitch
slow, low force of contraction, high resistance to fatigue, energy source: oxidative, red colour, function: posture, examples: anti-gravity muscles, e.g. abdominal muscles