Chemistry - Ch 8
5.0(1)
5.0(1)
Card Sorting
1/30
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
1
New cards
1
mono
2
New cards
2
di
3
New cards
3
tri
4
New cards
4
tetra
5
New cards
5
penta
6
New cards
6
hexa
7
New cards
7
hepta
8
New cards
8
octa
9
New cards
9
nona
10
New cards
10
deca
11
New cards
Bond-dissociation energy
amount of energy needed to break bonds
12
New cards
Inverse relationship
bond length and bond energy
13
New cards
Bond length
the distance between two bonded nuclei
14
New cards
Ionic bonds
a metal and a non-metal, transfer electrons
15
New cards
Covalent/Molecular bonds
a non-metal and a non-metal, share electrons
16
New cards
unshared pairs of electrons influence:
molecular shape
17
New cards
Lewis structures can *predict*:
molecular shape
18
New cards
Valence electrons determine:
shape
19
New cards
0 - 0.5
nonpolar covalent
20
New cards
0\.5 - 2.1
polar covalent
21
New cards
2\.1 - 3.3
ionic
22
New cards
Polarity is related to:
Bond Strength
23
New cards
Linear
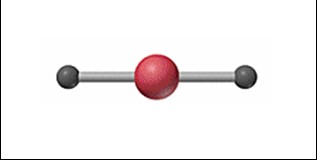
24
New cards
Bent
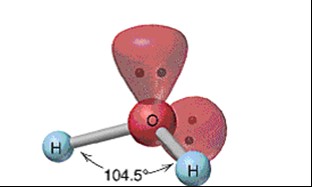
25
New cards
Trigonal Planar
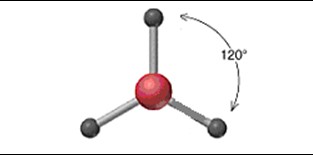
26
New cards
Trigonal Pyramidal
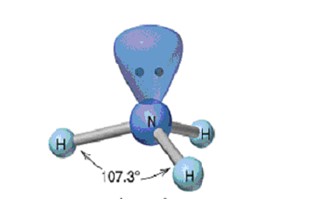
27
New cards
Tetrahedral
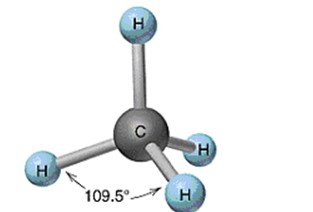
28
New cards
Diatomic Molecules
N, H, O, Cl, F, Br, I
29
New cards
A region of high probability where electrons are shared
Molecular orbital
30
New cards
Molecular bonds lengths are averages (because:)
The bonds are flexible
31
New cards
Which atom is most likely to form a triple covalent bond
Carbon