2.2 Electrons, bonding and structure
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
What is a shell in atomic structure? (1)
A group of orbitals with the same quantum number
What are the sub-shells in an atom? (4)
- s
- p
- d
- f
What is an atomic orbital? (2)
- A region around the nucleus that can hold up to two electrons
- With opposite spins
Why do electrons pair with opposite spins? (1)
To make the atom stable
What shape does an s orbital have? (1)
Spherical
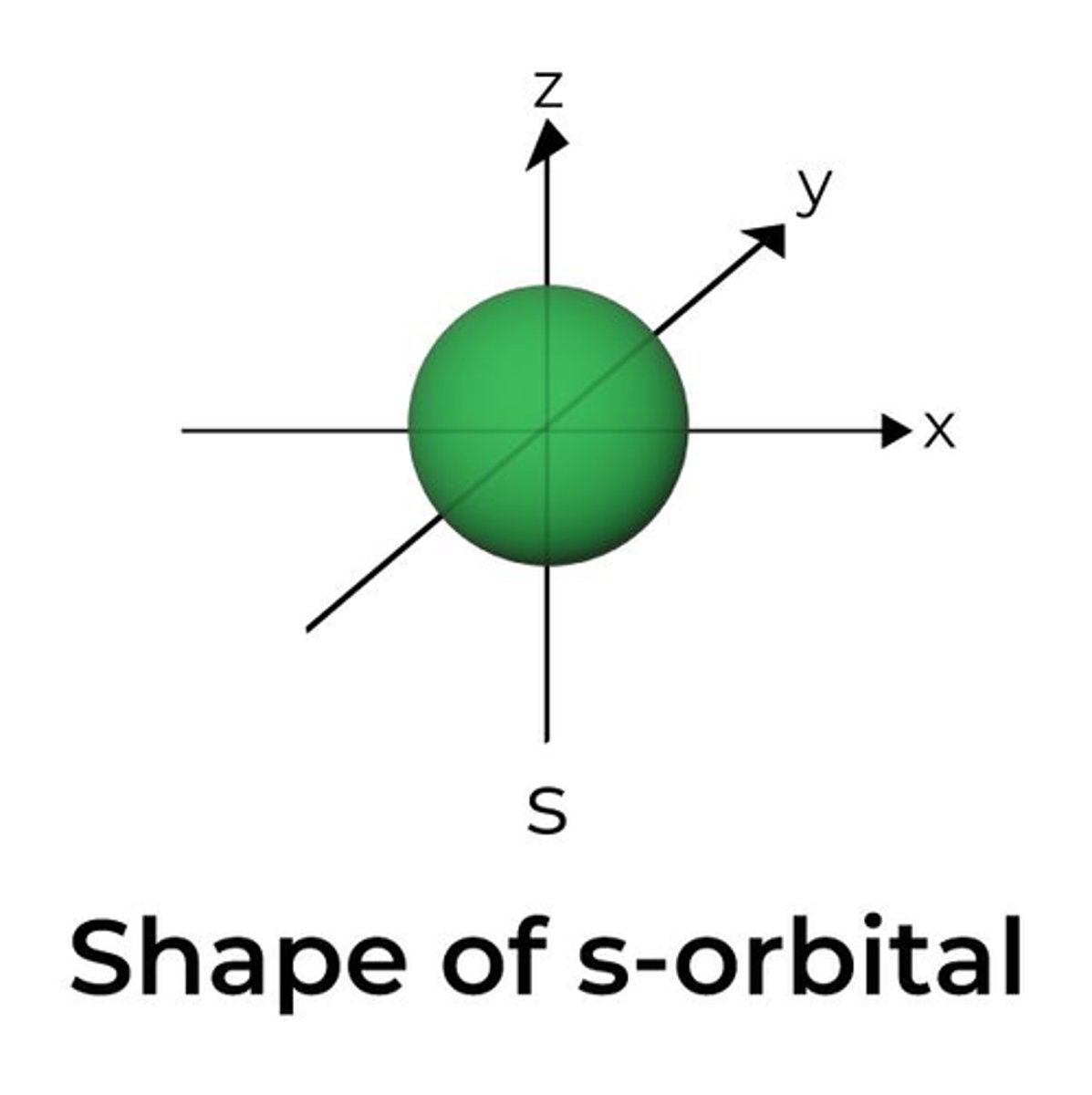
What shape does a p orbital have?
Dumbbell shape

What is the maximum number of electrons a shell can hold? (1)
2n², where n is the shell number.
How many orbitals are in each sub-shell? (3)
- s sub-shell: 1 orbital
- p sub-shell: 3 orbitals
- d sub-shell: 5 orbitals
How many electrons can one orbital hold? (1)
2 electrons
What sub-shells are found in the first electron shell? (1)
1s
What is the maximum number of electrons in the first electron shell? (1)
2
What sub-shells are found in the second electron shell? (1)
- 2s
- 2p
What is the maximum number of electrons in the second electron shell? (1)
8
What sub-shells are found in the third electron shell? (3)
- 3s
- 3p
- 3d
What is the maximum number of electrons in the third electron shell? (1)
18
How does the energy of orbitals change from s to f? (1)
Energy increases in the order s < p < d < f.
What happens to the energy of a shell as it gets further from the nucleus? (1)
It increases in energy
What is the exception to the usual energy order of orbitals? (1)
4s has a lower energy than 3d, so 4s is filled first.
Do all orbitals of the same type have the same energy? (1)
- Yes
EXAMPLE: Each 2p orbital has the same energy
What is the rule for filling orbitals with electrons? (1)
Orbitals are filled in order of increasing energy
How do electrons fill orbitals of the same energy? (1)
Electrons fill orbitals singly before pairing up
What is the 'electrons in box' representation of electronic structure? (3)
- Each box represents an orbital
- Boxes are arranged by increasing energy, degenerate orbitals are shown next to each other
- Opposite single headed arrows show electrons of opposite spins
What is the sub-shell notation for electronic structure?
- Notation which describes the arrangement of electrons within an atom's shells
- Specifying the shell number,
- Specifies the sub-shell type (s, p, d, f),
- States the number of electrons in that sub-shell
EXAMPLE: Oxgen: 1s2 2s2 2p4
What are the two exception exceptions to regular electronic configurations? (2)
- Chromium
- Copper
What is the sub-shell notation for chromium? (1)
Cr: 1s2 2s2 2p6 3s2 4s1 3d5
What is the sub-shell notation for copper? (1)
Cu: 1s2 2s2 2p6 3s2 3p6 4s1 3d10
Why are chromium and copper exceptions to regular electronic configurations? (2)
- For Cr and Cu, the 4s and 3d orbitals lie very close in energy
- It is energetically favourable to have half or completely filled sub-shells
What is ionic bonding? (1)
A strong electrostatic attraction between oppositely charged ions
How do ionic bonds form? (1)
Electrons are transferred from the metal to the non-metal to achieve full outer shells
What structure do ionic compounds form? (2)
- A giant ionic lattice
- With regular structural arrangements
How can dot-and-cross diagrams be used to show the electronic structure of ionic compounds? (2)
- Dots and crosses can be used to represent electrons from each species
- Transfer of electrons between species can be visualised
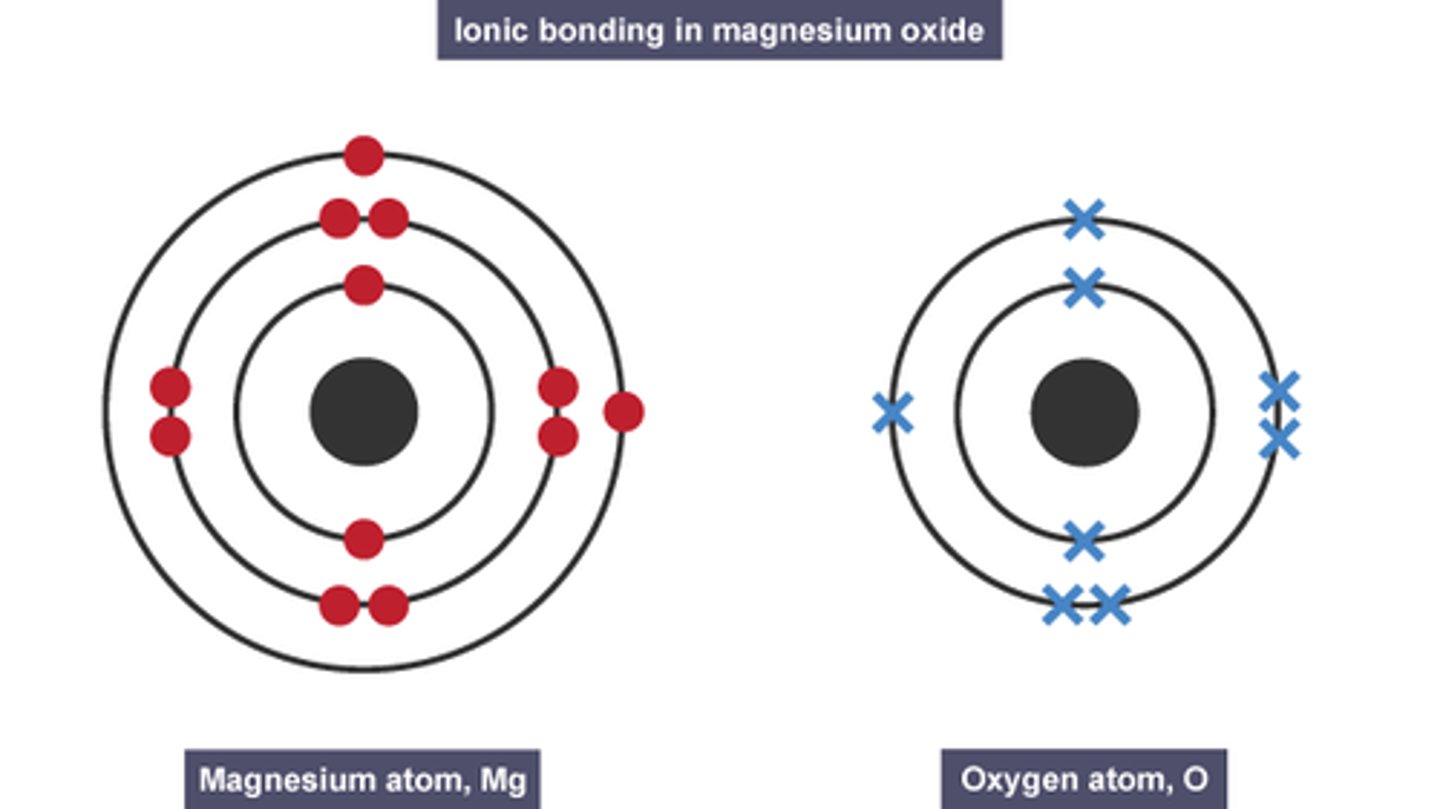
Why do giant ionic lattices have high melting and boiling points? (2)
- A lot of energy is needed to overcome strong electrostatic forces between oppositely ions.
- The greater the charge, the stronger the electrostatic force, leading to a higher melting point.
Why are ionic compounds soluble in polar solvents? (3)
- Slightly positive cations attract anions of the compound
- Slightly negative anions attract cations, disrupting and dissolving the lattice
- The solvent disrupts and completely surrounds the lattice, pulling it apart so it dissolves
Why do ionic compounds not conduct electricity as solids? (1)
Ions are fixed in place and cannot move.
When do ionic compounds conduct electricity? (2)
- When molten or in solution,
- Because ions are free to move and carry charge.
What is a covalent bond? (1)
A strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms.
How do covalent bonds form? (1)
Electrons are shared between non-metals to achieve a full outer shell.
How should dot-and-cross diagrams be drawn for covalent molecules? (1)
Draw atoms overlapping with at least one pair of electrons in the overlapping area.
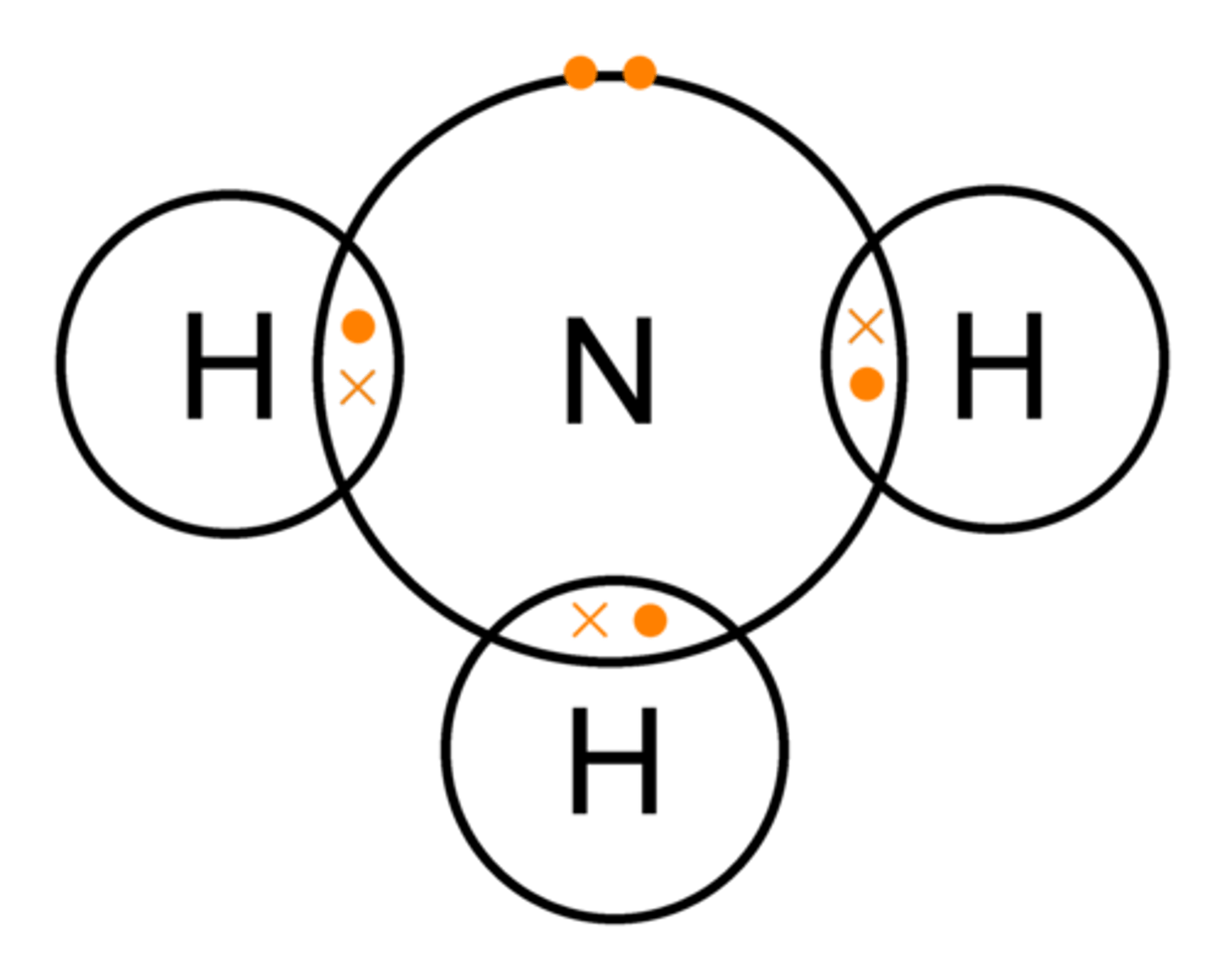
What is single covalent bonding? (1)
A covalent bond where only one pair of electrons is shared between atoms.
EXAMPLE: NH3
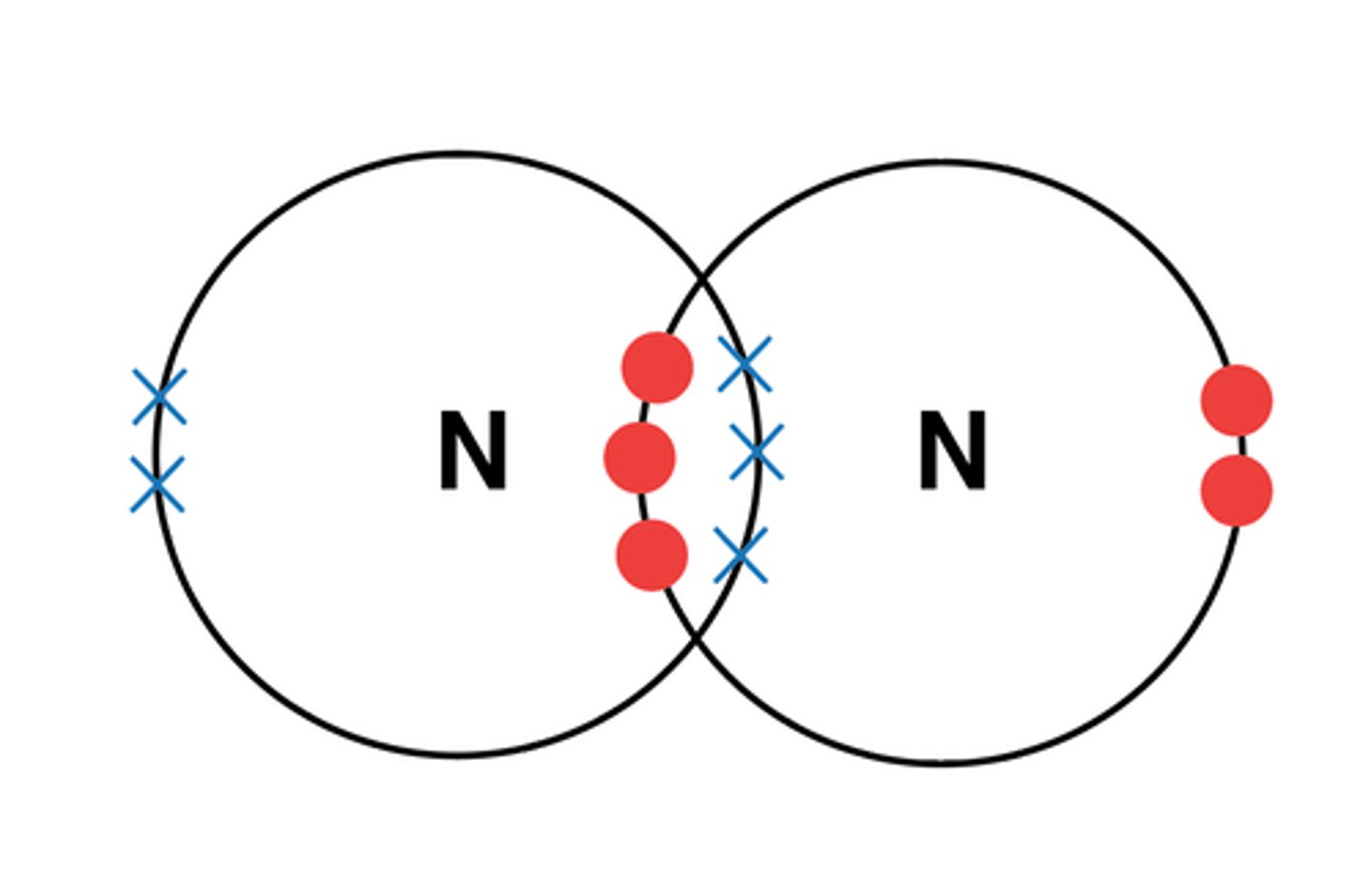
What is multiple covalent bonding? (1)
A covalent bond where more than one pair of electrons is shared between atoms.
EXAMPLE: N2
What is dative covalent (coordinate) bonding? (1)
A covalent bond in which a shared pair of electrons have both been donated by one atom.
What is average bond enthalpy? (2)
- Average bond enthalpy measures the strength of a covalent bond
- Indicates how much energy is required to break the bond
What is the relationship between average bond enthalpy and strength of a covalent bond? (1)
The larger the average bond enthalpy, the stronger the covalent bond.
What affects the shapes of simple molecules and ions? (3)
- Lone pairs of electrons repel more than bonding pairs.
- The repulsion follows the order: bond pair-bond pair < lone pair-bond pair < lone pair-lone pair.
- Angles between bonding pairs are usually smaller as they are pushed by lone pair repulsion.
What is the shape of a molecule with 2 total electron pairs? (2)
- Linear
- Bond angle: 180°
What is the shape of a molecule with 3 total electron pairs and 0 lone pairs? (2)
- Trigonal planar
- Bond angle: 120°
What is the shape of a molecule with 4 total electron pairs and 0 lone pairs? (2)
- Tetrahedral
- Bond angle: 109.5°
What is the shape of a molecule with 4 total electron pairs and 1 lone pair? (2)
- Pyramidal
- Bond angle: 107°
What is the shape of a molecule with 4 total electron pairs and 2 lone pairs? (2)
- Non-linear
- Bond angle: 104.5°
What is the shape of a molecule with 6 total electron pairs and 0 lone pairs? (1)
- Octahedral
- Bond angle: 90°
What is the shape bond angle for the CH4 molecule? (2)
- Tetrahedral
- 109.5°
What is the shape bond angle for the NH3 molecule? (2)
- Pyramidal
- 107°
What is the shape bond angle for the H2O molecule? (2)
- Non-linear
- 104.5°
What is electronegativity? (1)
The ability of an atom to attract the bonding electrons in a covalent bond.
What scale is electronegativity measured against? (2)
- The Pauling scale.
- The higher the value, the higher the electronegativity.
How does electronegativity change across the periodic table? (2)
- Electronegativity increases towards fluorine.
- Electronegativity increases across the period and decreases down the groups.
What is a non-polar bond? (2)
- A bond where the bonded electron pair is shared equally between the bonded atoms
- Because the bonded atoms have the same electronegativity.
What is a polar bond? (1)
- A bond where the bonded electron pair is shared unequally between the bonded atoms
- Because the bonded atoms have different electronegativity values.
What is a permanent dipole? (3)
- A separation of charge in a polar bond
- Where one atom has a slightly positive (δ⁺) charge and the other has a slightly negative (δ⁻) charge.
- The atom with greater electronegativity has the negative charge.
- The dipole moment is from δ⁺ to δ⁻
What is the dipole moment? (1)
The dipole moment is from δ⁺ to δ⁻

What is the relationship between dipole moment and electronegativity? (2)
The greater the difference in electronegativity, the greater the dipole moment.
How can you determine if a molecule is polar or non-polar? (2)
- A non-polar molecule has no polar bonds, or has polar bonds of which the dipoles cancel out due to the symmetry of the molecule.
- A polar molecule has polar bonds and an asymmetrical shape so the dipoles do not cancel.
What are intermolecular forces? (1)
Weak interactions between dipoles of different molecules.
What are the types of intermolecular forces from weakest to strongest? (1)
- Induced dipole-dipole interactions (London forces) (Weakest)
- Permanent dipole-dipole interactions
- Hydrogen bonding (strongest)
What are induced dipole-dipole interactions (London forces)? (4)
- Occurs between all molecules.
- Constant random movement of electrons causes an uneven distribution of charge, leading to a temporary dipole,
- Temporary dipoles induce dipoles in neighbouring molecules.
- The larger the relative molecular mass (Mr), the stronger the London forces because it has more electrons.
What are permanent dipole-dipole interactions? (2)
- Between polar molecules.
- Molecules with permanent dipoles attract other molecules with permanent dipoles.
What IMF can be classified as van der Waals' forces? (2)
- Permanent dipole-dipole interactions
- Induced dipole-dipole interactions
What is hydrogen bonding? (2)
- A strong permanent dipole-dipole interaction.
- Occurs between H⁺ and a lone pair of electrons on a highly electronegative atom (O, N, F)
How do intermolecular forces affect melting and boiling points? (2)
- When melting and boiling, intermolecular forces are overcome.
- Stronger forces require more energy to overcome, causing higher melting and boiling points.
What are the effects of hydrogen bonding in water? (3)
- Water has high melting and boiling points compared to molecules of a similar size.
- Ice is less dense than water because molecules in ice are held apart by hydrogen bonds in an open lattice.
- The hydrogen bonds break when ice melts.
What are simple molecular lattices? (2)
- Covalently bonded molecules
- Attracted to neighbouring molecules by intermolecular forces
Why do covalent compounds have low melting and boiling points? (2)
- Molecules are held together by weak intermolecular forces
- Only a small amount of energy is required to overcome them.
Why do covalent compounds not conduct electricity? (2)
- Simple molecular lattices have no delocalised electrons
- So cannot carry a charge
Why are covalent compounds often soluble in non-polar solvents? (2)
- Intermolecular forces are able to form between the solute and solvent
- These forces help to break down the simple molecular lattice