C2b Electrolysis
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Why can ionic compounds not conduct electricity when solid?
The ions are not free to move
Why can ionic compound conduct electricity when molten or aqueous?
The ions are free to move
What is an electrolyte?
An ionic compound that is aqueous or molten and so able to conduct electricity
What is a cation?
A positively charged ion
What is an anion?
A negatively charged ion
What charge is the cathode?
Negative
What charge is the anode?
Positive
What ions move towards the cathode?
Positively charged ions (cations)
What ions move towards the anode?
Negatively charged ions (anions)
Why do cations move towards the cathode?
The cathode is negatively charged and opposite charges attract
Why do anions move towards the anode?
The anode is positively charged and opposite charges attract
What is the produced at the cathode when aqueous solutions undergo electrolysis? (2)
If the metal is more reactive than hydrogen, then hydrogen gas (H2) is produced
If the metal is less reactive than hydrogen then the metal is produced
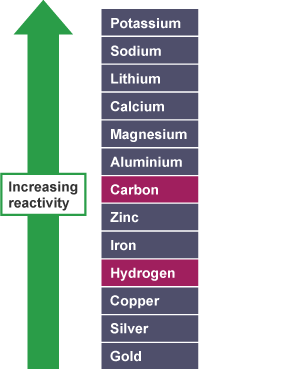
What is produced at the anode when aqueous solution undergo electrolysis? (2)
If halide ions (group 7: halogens) are present, then the respective halogen is produced
If not then oxygen is produced
What is bauxite?
Aluminium oxide (Al2O3)
Why is cryolite added when aluminium oxide (bauxite) undergoes electrolysis?
Lower the melting point
During the electrolysis of aluminium oxide (bauxite), why does the anode need to be regularly replaced?
Oxygen is produced at the anode which reacts with the carbon anode to produce carbon dioxide