1.1. ATOMIC STRUCTURE
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
How are mass and charge distributed in an atom?
Mass is concentrated in the nucleus (protons and neutrons).
Electrons contribute very little to mass but create a cloud of negative charge.
Electrostatic attraction between the positive nucleus and negative electrons holds the atom together.
How do protons, neutrons, and electrons behave in an electric field?
Electrons: Deflected strongly toward the positive plate (light and negatively charged).
Protons: Deflected weakly toward the negative plate (heavier and positively charged).
Neutrons: Not deflected (neutral charge).
How can you determine the numbers of protons, neutrons, and electrons in atoms and ions?
Protons: Equal to the atomic number (Z).
Neutrons: Mass number (A) - Atomic number (Z).
Electrons:
Neutral atom: Same as protons.
Positive ion: Fewer electrons than protons.
Negative ion: More electrons than protons.
What is atomic radius, and how does it vary across a period and down a group?
Definition: Atomic radius is half the distance between the nuclei of two covalently bonded atoms of the same type.
Across a Period:
Atomic radius decreases due to increased positive nuclear charge pulling electrons closer.
Extra electrons are added to the same principal quantum shell, enhancing nuclear attraction.
Down a Group:
Atomic radius increases due to additional electron shells.
Inner electrons shield outer electrons from the positive nucleus, weakening nuclear pull.
How does the electron shell theory explain atomic radius trends?
Across a Period:
Increased nuclear charge pulls electrons closer, reducing atomic size.
Down a Group:
More electron shells increase shielding, reducing nuclear attraction and increasing atomic size.
Special Case:
Atomic radius increases sharply between noble gases and alkali metals due to an extra principal quantum shell.
What is ionic radius, and how does it vary with charge?
Definition: Ionic radius is the size of an ion.
Negative Ions:
Formed by gaining electrons.
Increased electron repulsion weakens nuclear pull, increasing ionic radius.
Greater negative charge → Larger ionic radius.
Positive Ions:
Formed by losing electrons.
Fewer electrons experience stronger nuclear attraction, decreasing ionic radius.
Greater positive charge → Smaller ionic radius.
How does the electron shell theory explain ionic radius trends?
Negative Ions:
Extra electrons increase repulsion, weakening nuclear pull and enlarging the ion.
Positive Ions:
Fewer electrons experience stronger attraction, shrinking the ion.
What are isotopes?
Isotopes are atoms of the same element with the same number of protons and electrons but a different number of neutrons.
Example:
Carbon-12 (6 neutrons) and Carbon-14 (8 neutrons) are isotopes of carbon.
Isotopes are named using the chemical symbol followed by a dash and the mass number (e.g., Carbon-12).
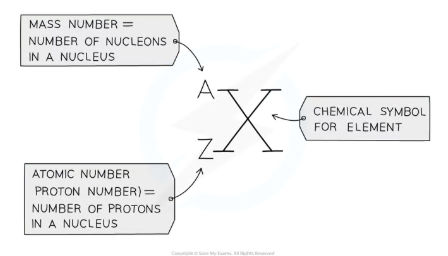
How are isotopes represented using notation?
x = Mass number (protons + neutrons). y = Atomic number (protons). A = Chemical symbol.
Example:
Chlorine-35: Mass number = 35, Atomic number = 17, Chemical symbol = Cl.
Chlorine-37: Mass number = 37, Atomic number = 17, Chemical symbol = Cl.
Why do isotopes of the same element have the same chemical properties?
Isotopes have the same number of electrons in their outer shells.
Electrons determine chemical reactions, so isotopes of the same element behave identically in chemical processes.
Why do isotopes of the same element have different physical properties?
Isotopes differ in the number of neutrons, which affects their mass and density.
Neutrons are neutral and only add mass to the atom.
Example:
Chlorine-35 has a lower mass and density than Chlorine-37 due to fewer neutrons
What are shells, sub-shells, and orbitals in an atom?
Shells: The main energy levels around the nucleus, numbered using principal quantum numbers (n).
n = 1 (up to 2 electrons), n = 2 (up to 8 electrons), n = 3 (up to 18 electrons), n = 4 (up to 32 electrons).
Sub-shells: Divisions of shells, labeled as s, p, d, and f. Their energy increases as s < p < d < f.
Example: n = 1 only has an s sub-shell, while n = 3 has s, p, and d sub-shells.
Orbitals: Specific regions in sub-shells where electrons are found.
s: 1 orbital (2 electrons), p: 3 orbitals (6 electrons), d: 5 orbitals (10 electrons), f: 7 orbitals (14 electrons).
What is the principal quantum number (n) and how does it relate to shells?
The principal quantum number (n) indicates the energy level of a shell.
Lower n = closer to the nucleus and lower energy.
Higher n = farther from the nucleus and higher energy.
It also determines the maximum number of electrons in a shell:
n = 1 (2 electrons), n = 2 (8 electrons), n = 3 (18 electrons), n = 4 (32 electrons).
What is the ground state of an atom?
The ground state is the most stable electronic configuration with the lowest energy.
Electrons fill sub-shells in order of increasing energy, starting with the 1s orbital.
Example: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p.
How many orbitals and electrons are in each type of sub-shell (s, p, d)?
s sub-shell: 1 orbital, 2 electrons.
p sub-shell: 3 orbitals, 6 electrons.
d sub-shell: 5 orbitals, 10 electrons.
f sub-shell (not at AS level): 7 orbitals, 14 electrons.
What is the order of increasing energy in sub-shells?
Energy generally increases as follows: s < p < d < f.
The 4s sub-shell is lower in energy than the 3d sub-shell, so it is filled first.
Example: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p.
How do you describe an atom's electronic configuration?
It lists the number of electrons in each shell and sub-shell.
Example for potassium (19 electrons): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
Box notation uses arrows in boxes to show individual electron spin.
What determines the energy of electrons and inter-electron repulsion?
Energy of electrons: Electrons fill the orbitals in order of increasing energy (e.g., 1s < 2s < 2p < 3s < 3p < 4s < 3d).
Inter-electron repulsion:
Electrons have spin, either clockwise or anticlockwise, and repulsion occurs between electrons with similar spin (spin-pair repulsion).
To minimize repulsion, electrons occupy separate orbitals within the same sub-shell first. Pairing occurs only when no empty orbitals are available.
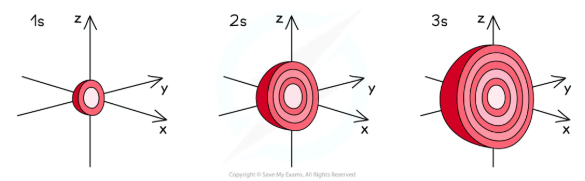
Shape of s Orbitals
The s orbitals are spherical in shape
The size of the s orbitals increases with increasing shell number
E.g. the s orbital of the third quantum shell (n = 3) is bigger than the s orbital of the first quantum shell (n = 1)
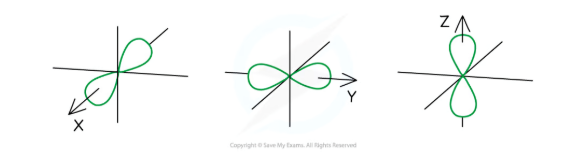
Shape of p Orbitals
The p orbitals are dumbbell-shaped
Every shell has three p orbitals except for the first one (n = 1)
The p orbitals occupy the x, y and z-axis and point at right angles to each other so are oriented perpendicular to one another
The lobes of the p orbitals become larger and longer with increasing shell number
How can you determine the electronic configuration of atoms and ions using full and shorthand notations?
Full notation: Lists all electrons from 1s onwards, e.g., Fe: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s².
Shorthand notation: Uses the nearest preceding noble gas to simplify, e.g., Fe: [Ar] 3d⁶ 4s².
Ions:
Negative ions: Add electrons to the outer sub-shell.
Positive ions: Remove electrons from the outer sub-shell. Transition metals lose electrons from the 4s sub-shell first, not the 3d sub-shell.
What is electrons in boxes notation, and how is it used?
Electrons in boxes: Represents orbitals as boxes arranged by increasing energy, with electrons shown as arrows indicating spin.
Example: For Fe ([Ar] 3d⁶ 4s²):
4s: ↑↓
3d: ↑↓ ↑↓ ↑↓
What is a free radical, and how is it represented?
Definition: A species with one or more unpaired electrons.
Representation: Unpaired electron shown as a dot. Example: Chlorine radical (1s² 2s² 2p⁶ 3s² 3p⁵).
Two p orbitals have paired electrons, and one p orbital has the unpaired electron.
Formation: Free radicals are formed via homolytic fission, where a covalent bond splits evenly, sharing electrons between two atoms.
What is the first ionisation energy (IE1), and how is it defined?
Definition: First ionisation energy is the energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous 1+ ions under standard conditions (298 K, 101 kPa).
Equation for IE1 (Example – Calcium):
Ca (g)→Ca+(g)+e−
IE1=+590 kJ mol−1
Key Points:
Ionisation energy is always positive as energy is required to overcome the attraction between the electron and the positive nucleus (endothermic process).
Units: kJ mol−1
How do you write equations for second and subsequent ionisation energies?
Second Ionisation Energy (IE2): Energy required to remove one mole of electrons from one mole of gaseous 1+ ions to form one mole of gaseous 2+ ions.
Equation: Ca+(g)→Ca2+(g)+e−
IE2=+1145.4 kJ mol−1
Subsequent Ionisation Energies: Follows the same pattern, with increasing energy required for each successive electron removal due to the increasing positive charge on the ion.
What are the trends in ionisation energies across a period and down a group?
Across a Period (Left to Right):
Increase in IE1 due to:
Increasing nuclear charge: Greater attraction between nucleus and electrons.
Decreasing atomic radius: Outer shell is closer to the nucleus.
Constant shielding: Electrons are added to the same shell.
Exceptions: Slight decreases due to sub-shell effects (e.g., spin-pair repulsion in Oxygen vs. Nitrogen).
Down a Group (Top to Bottom):
Decrease in IE1 due to:
Increased atomic radius: Outer electrons are farther from the nucleus.
Increased shielding: Inner electrons reduce nuclear attraction.
These factors outweigh the increasing nuclear charge.
What causes variations in successive ionisation energies of an element?
Successive Ionisation Energies Increase:
Removing electrons from a positive ion requires more energy.
Less shielding and a higher proton-to-electron ratio increase nuclear attraction.
Sharp Increase: Occurs when electrons are removed from an inner, more stable shell closer to the nucleus.
Why are ionisation energies due to the attraction between the nucleus and the outer electron?
Outer electrons are attracted by the positive charge of the nucleus.
Factors affecting nuclear attraction:
Nuclear charge: More protons = stronger attraction.
Atomic radius: Larger distance = weaker attraction.
Shielding: Inner electrons reduce the effective nuclear charge felt by outer electrons.
What factors influence the ionisation energy of an element?
Nuclear Charge: More protons = stronger attraction to outer electrons = higher IE.
Atomic/Ionic Radius: Greater distance from nucleus = weaker attraction = lower IE.
Shielding by Inner Shells/Sub-shells: More inner electrons = greater shielding = lower IE.
Spin-Pair Repulsion: Electrons in the same orbital repel each other, making it easier to remove one = lower IE.
How can successive ionisation energy data be used to deduce the electronic configuration of elements?
Successive Ionisation Energies:
Successive ionisation energy values increase as more electrons are removed.
Large increases in ionisation energy occur when removing an electron from a full shell (closer to the nucleus).
Deducing Electronic Configuration:
Identify where the sharp increase in energy occurs.
The number of electrons removed before the sharp increase corresponds to the number of electrons in the outer shell.
Example (Calcium):
Successive ionisation energies: 590, 1145, 4940 kJ mol⁻¹.
Sharp increase after removing 2 electrons indicates the element has 2 outer electrons → group 2 element.
Electronic configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s².
How can successive ionisation energy data be used to deduce the position of an element in the Periodic Table?
Outer Electrons & Group Number:
The number of electrons in the outer shell (found from the sharp increase) determines the element's group.
Example: Two outer electrons → group 2.
Number of Electron Shells & Period Number:
The number of occupied shells corresponds to the period.
Successive ionisation energy data can show the energy levels of electrons, indicating the number of shells.
Example:
Successive ionisation data: 800, 2427, 3660, 25000 kJ mol⁻¹.
Sharp increase after 3 electrons → group 3 (outer configuration: 3 electrons).
Ionisation energies indicate 3 shells → period 3.
Element: Aluminium (Al).
What factors influence successive ionisation energy data?
Nuclear Charge:
Higher proton number → stronger attraction to electrons → higher ionisation energy.
Shielding:
Inner electrons reduce the effective nuclear charge felt by outer electrons → lower ionisation energy.
Atomic/Ionic Radius:
Larger radius → greater distance between nucleus and outer electron → lower ionisation energy.
Sub-Shell & Spin-Pair Repulsion:
Electrons in the same orbital repel each other → lower ionisation energy due to repulsion.