Cell Biology lecture 23
1/32
Earn XP
Description and Tags
Electro-osmotic effects in cells
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
LOs
Electro-osmotic effects in cells.
Free energy, chemical and electrical work across membranes.
Physical basis of the Nernst potential.
Membrane potential
Osmotic pressure and the sodium anomaly.
Chemiosmosis and the proton motive force.
why don’t cells don’t just burst?
Plants have strong cell walls to deal with turgor pressure but the imbalance of ions and large charged molecules in animals needs to be dealt with. Use chemiosmosis to help deal with this.
main components of charge in cell

what allows thermodynamic analysis of mixtures?
The chemical potential
what is the chemical potential?
= free energy + entropic effects
Potential of these chemicals to move

at equilibrium what happens to the chemical potentials
They must match for each permanent species
For a single permanent species this just means that concentrations balance out atequilibrium
What happens at a membrane when we have a mixture of permanent and impermanent ions - theory if both were permeable ?
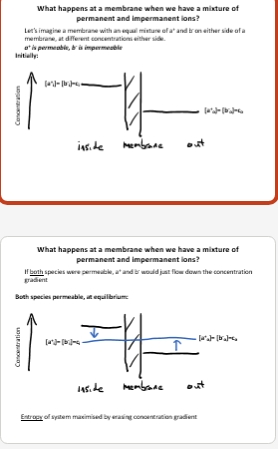
What happens at a membrane when we have a mixture ofpermanent and impermanent ions? - if just A+ permeable?
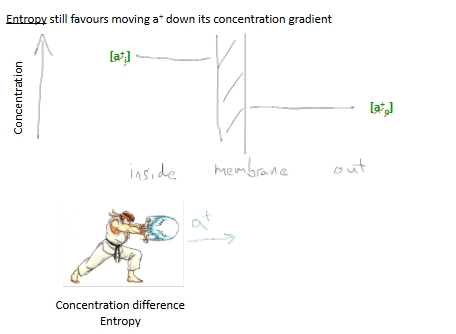
What happens at a membrane when we have a mixture ofpermanent and impermanent ions - if b- is impermeable?

what does this electric field do with transfer of permeable and impermeable ions?
The electric field set up by ion imbalance opposes transfer of further ions
what pushs permable ions in opp directions?
Conc gradient (entropy) and electric potential diff (enthalpy)

Nernst potential
electrical potential difference required to stop the flow of ions arising from a concentration difference
The potential difference due to charge transfer builds up enough to stop further flow of ions
No flow = equilibrium
This is the Nernst potential, ΔV = Vnernst
How do we calculate Nernst?
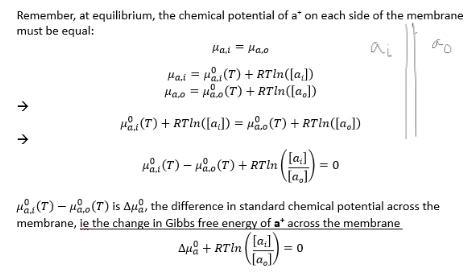
what does electric potential difference cause across the membrane?
causes a change in Gibbs free energy
change in internal energy = charge x change in potential diff
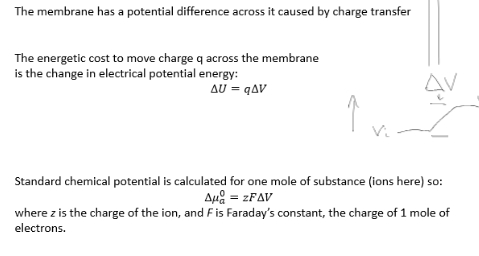
Nernst equation for conditions of equilibrium across the membrane (just use for understanding of equation)
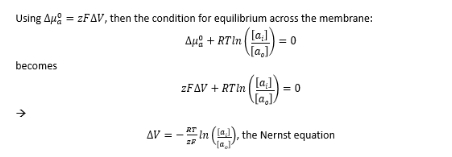
what is the Nernst potential
Voltage diff across the membrane created by transferring just enough permanent ions to create an electrostatic barrier against further flow of ions
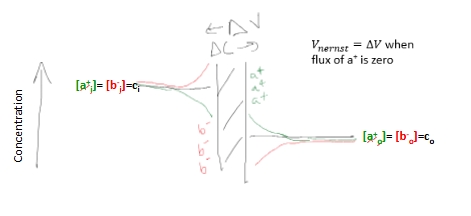
Nernst potential and cells
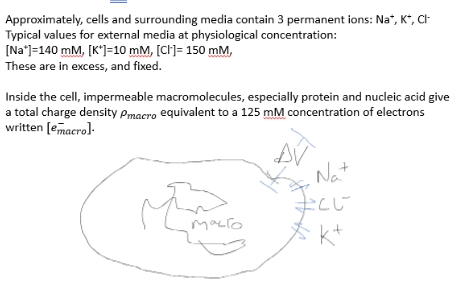
At equilibrium, what is the internal concentration of each ion and the potential difference across the membrane?
The external ion concentrations, and the internal macromolecule concentrations are fixed.
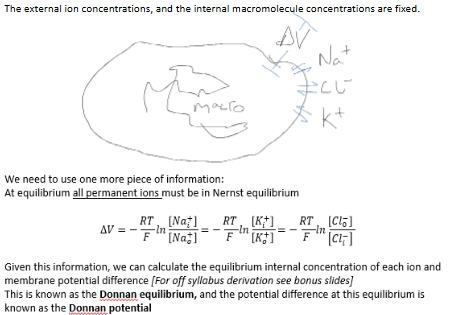
Donnan equilibrium
equilibrium internal conc of each ion and membrane potential diff
Donnan potential
potential difference for Donnan equilibrium
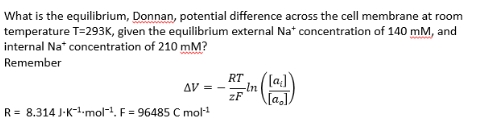
Calculating Donnan potentials
Answer = -10mV
Are cells at Donnan equilibrium?
If cells were at Donnan equilibrium, what would their osmotic pressure be?
How does the observed membrane potential match up with each ions Nernst potential?
What is the osmotic pressure of the cell at Donnan equilibrium?
osmotic pressure is a stretching force on the cell membrane, caused by an imbalance of stuff on either side. Too much osmotic pressure can burst the cell.
To be calc in next slides

What is the osmolarity difference for a cell at Donnan equilibrium?
Answer = 25mOsm
What is the osmotic pressure of the cell at Donnan equilibrium?

Observed ion concentrations and membrane potential are far from Donnan equilibrium
Pioneering electrophysiology measurements of membrane potential were performed by inserting electrodes in squid giant axons
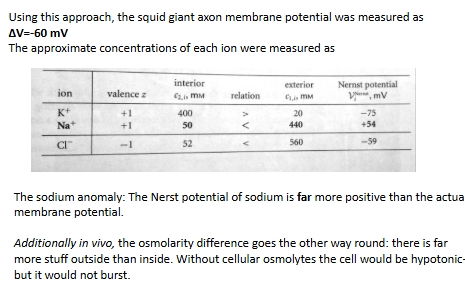
why is cell far from Donnan equilibrium?
This explains why cells don’t burst: the cells are nowhere near electrochemical equilibrium.
The cell avoids bursting by actively pumping Na+ and K+ ions.
Also, setting up such a strong imbalance allows the cell to transiently allow Na+ to flow back into the cell by opening ion channels.
Which is where the action potential comes from.
why discuss these potentials?
Basic electrochemistry shows us how membrane potentials arise
Cell membrane potentials are a great example of how quantitative analysis gives us the tools to understand how cells must work, ie how physical laws shape biological organisms
The pH of the cytosol is 7.4. The hydrogen ion concentration in mitochondria is 1.6nM.
What is the pH difference across the mitochondrial membrane ΔpH = pHin - pHout?
Remember, pH = -log([H+])
Answer = 1.4
Mitochondria as factories
Eukaryotic cells actively maintain a sodium ion imbalance to avoid bursting and, inneurons, to communicate
It turns out both mitochondria and bacteria use a similar strategy, but withhydrogen ions, to generate ATP, the energy currency of the cell.
Mitochondrial electrical imbalances and uses
Eukaryotic cells actively maintain a sodium ion imbalance to avoid bursting and, in neurons, to communicate
It turns out both mitochondria and bacteria use a similar strategy, but with hydrogen ions, to generate ATP, the energy currency of the cell.
Respiration sets up a large proton concentration (pH) imbalance
The chemiosmotic hypothesis: cellular ATP is generated using the mitochondrial proton motive force
ATP synthase is a rotary motor powered by proton flux
This generates ATP

What is another rotary motor powered by proton flux?
Bacterial flagellar motor
Summary
The chemical potential describes the thermodynamic behaviour of mixtures ofspecies
Ion gradients across semi-permiable membranes lead to membrane potentialdifference at equilibrium, the Nernst potential
Living cells establish membrane potential differences far away from the Nernstpotential by actively pumping ions across their membranes
Uses of membrane potential:
Prevent excessive osmotic pressure
Action potential
ATP synthesis
Rotation of the bacterial flagella (swimming)