Selectivity of electrophilic addition in alkenes
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
How does electrophilic addition change the hybridisation
The carbon atoms go from sp2 to sp3
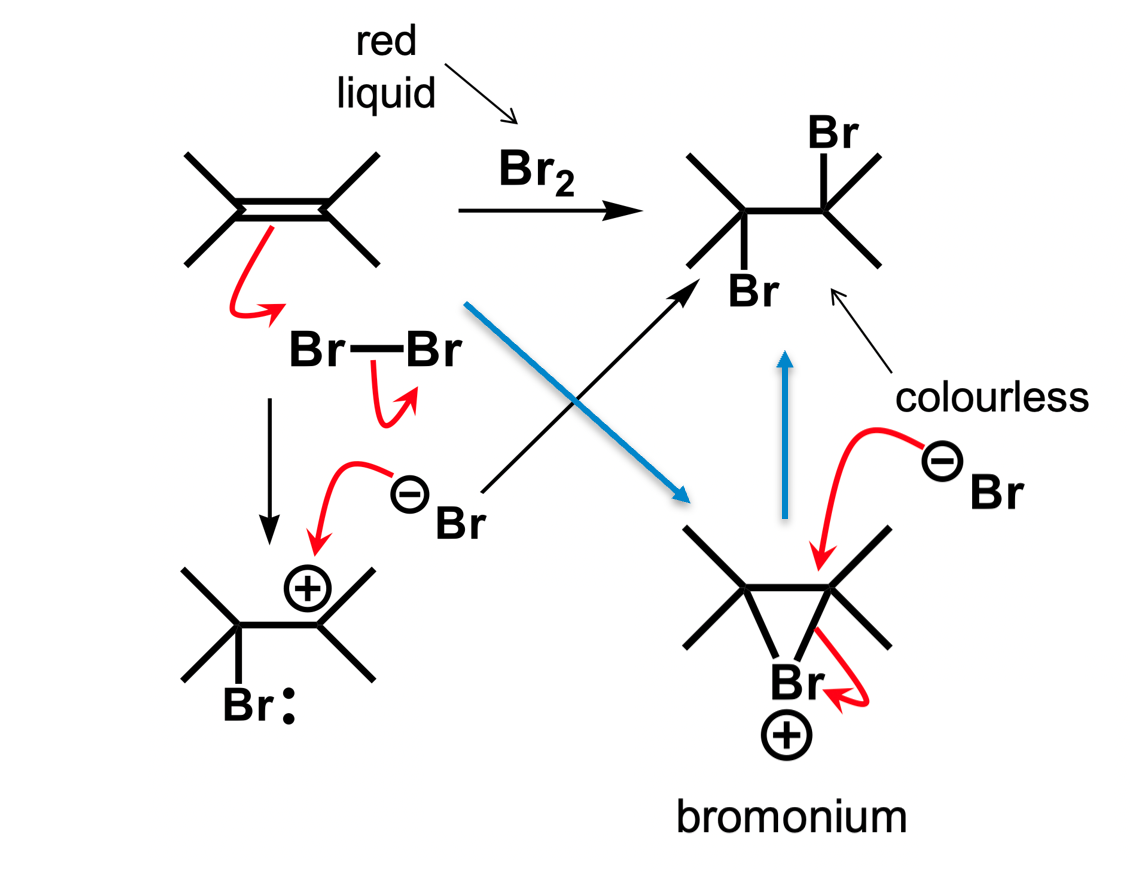
What is the actual mechanism for electrophilic addition of Br2 and what are the consequences of this
The mechanism follows the blue arrows. Since the bromonium ion must form in a cis- style conformation and the Br- attacks form the back of the bond, this reaction is stereospecific and will only form the trans- product.
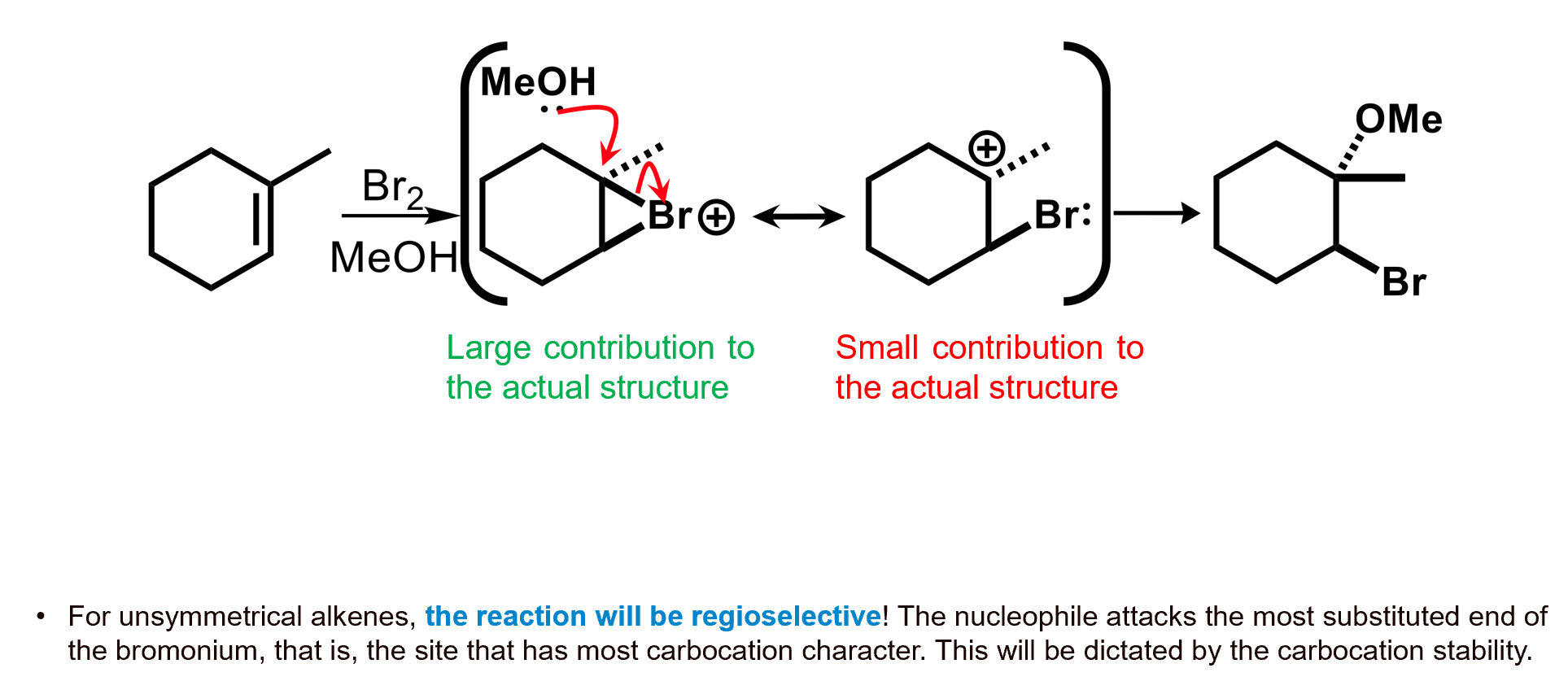
What about if there is another, stronger nucleophile than Br- present.
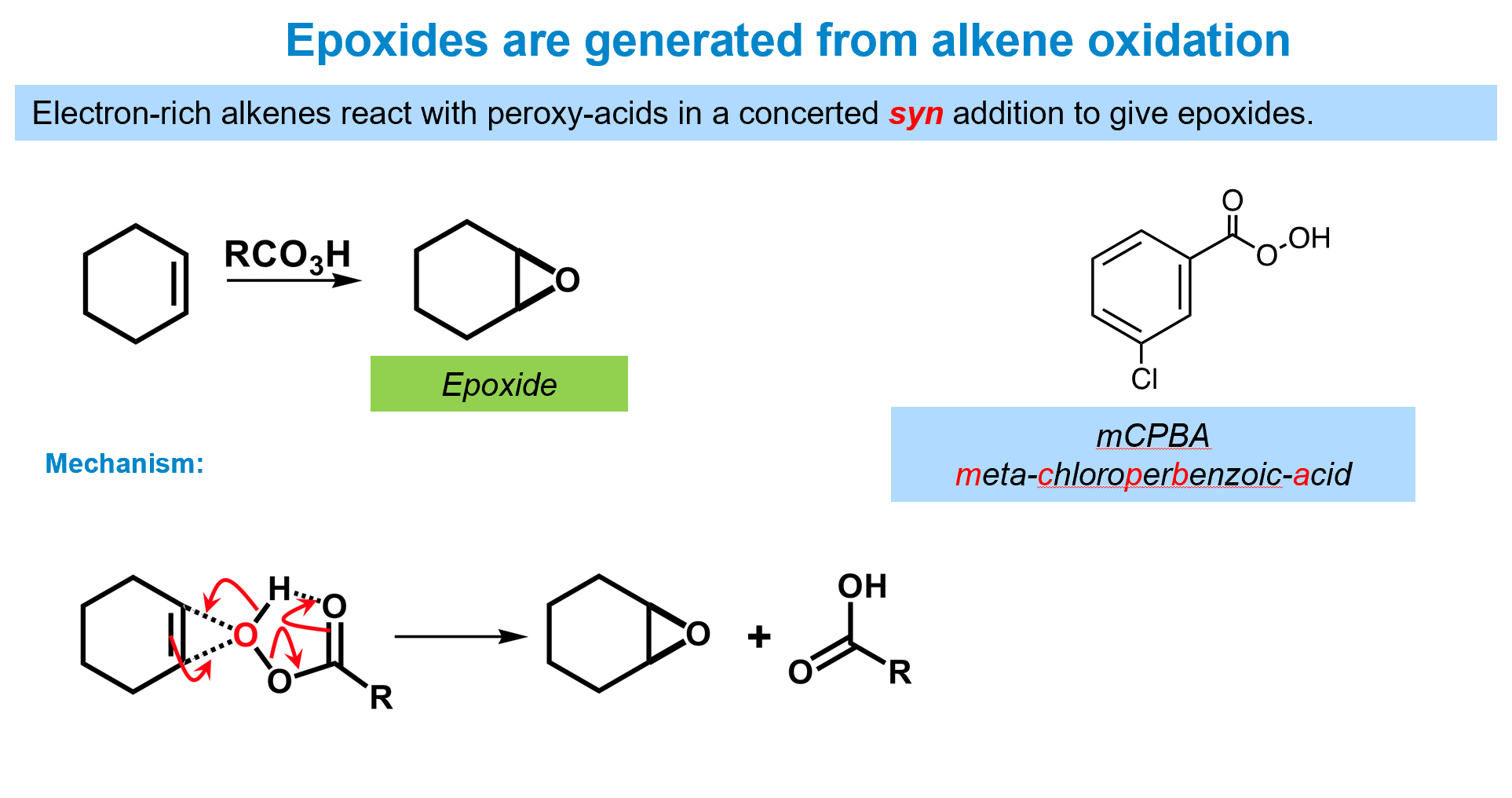
How can you form epoxides
the reactant used is a peracid - a carboxylic acid with an additional oxygen.
The reaction proceeds exactly like the bromonium one, forming an anti- product.
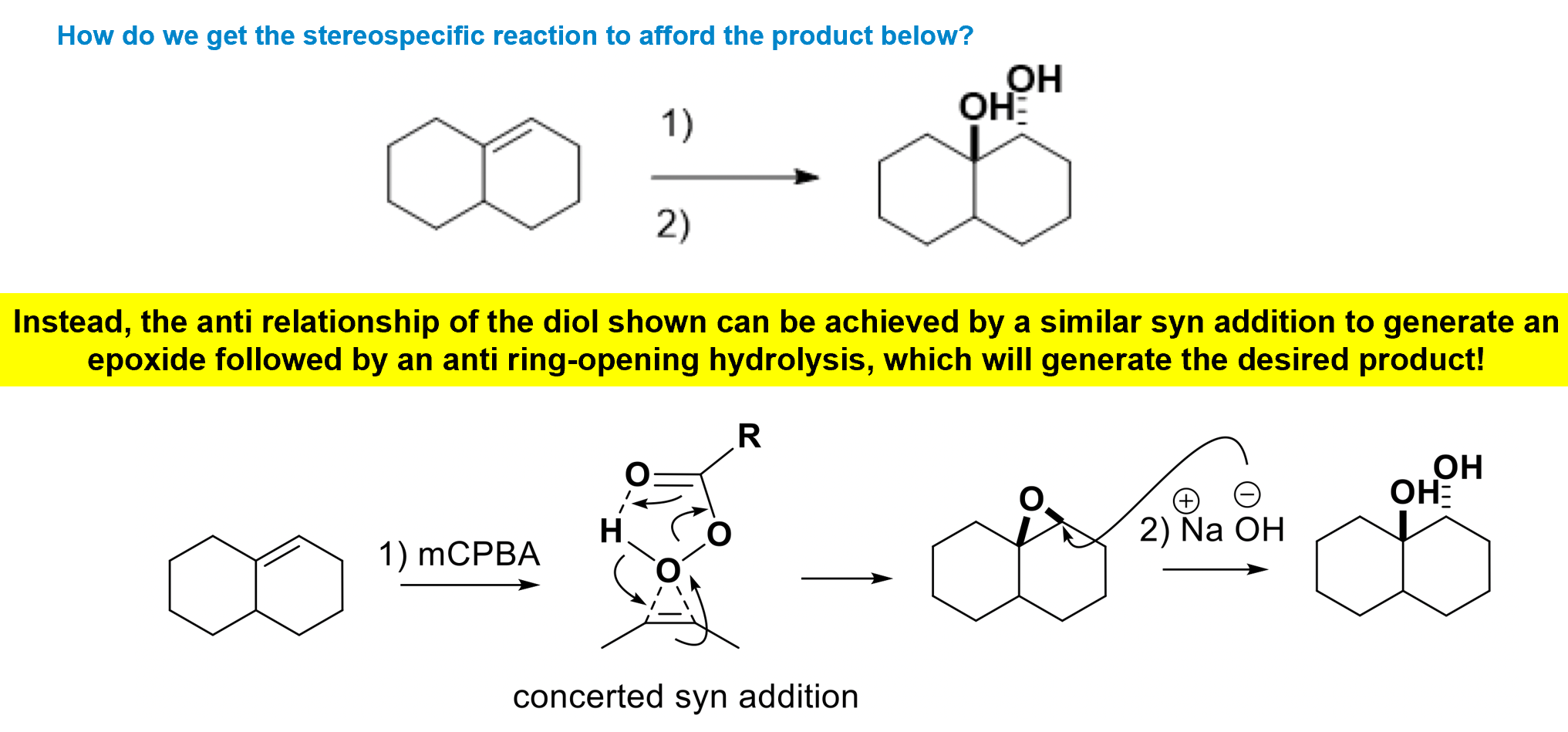
Anti-dihydroxylation of alkenes
We form an epoxide using a peracid and then use anti- attack of a nucleophile to generate an anti- diol.
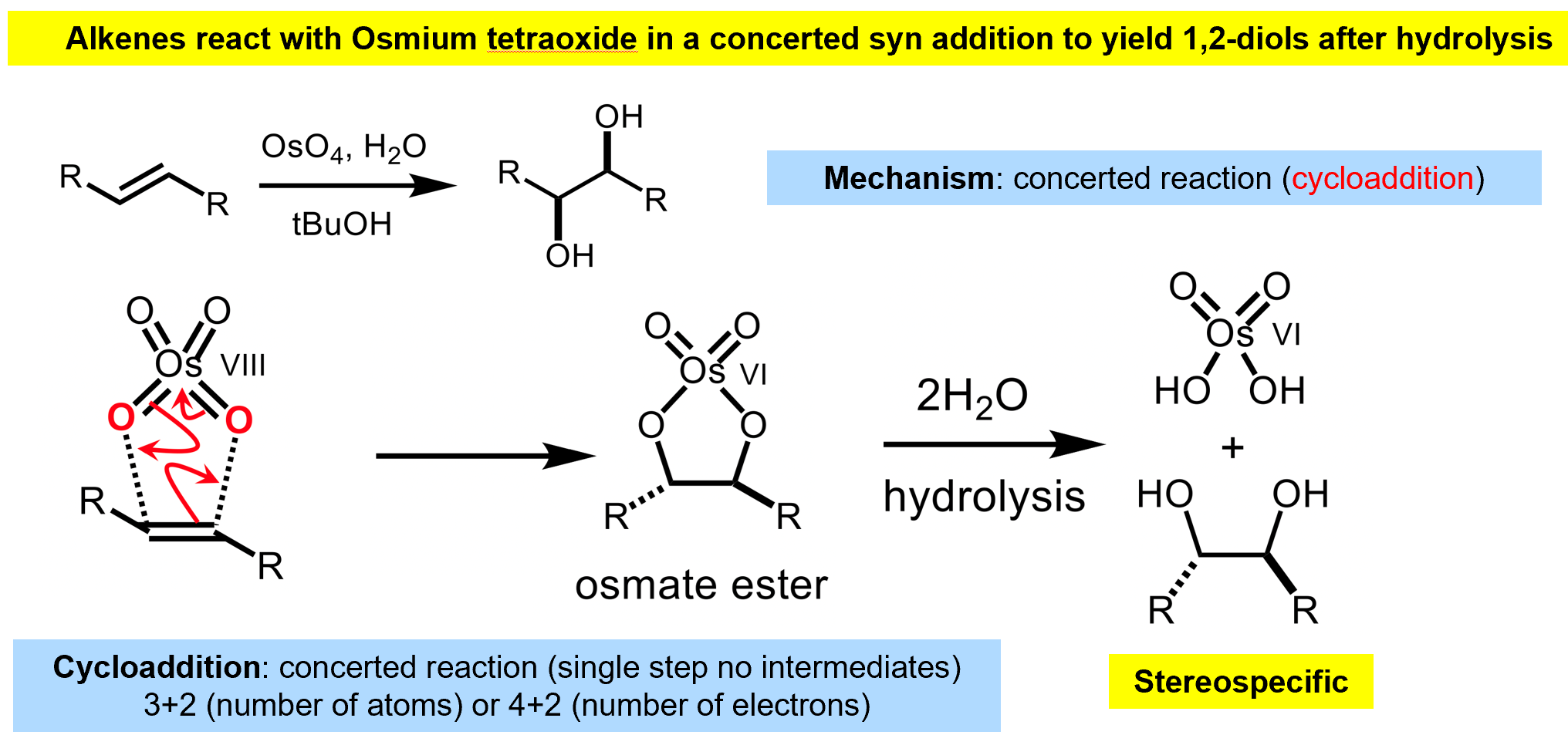
Syn-Dihydroxylation of alkenes
The osmate ester is analogous to a bromonium/epoxide however thanks to the osmate structure we will end up with a syn- product.