Structure and composition of the atmosphere
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What is the atmosphere?
Thin layer of gases surrounding the earth, held in place by gravity
How is it essential to life on earth?
Provides a vital support system:
Source of gases for natural processes
Absorbs electromagnetic radiation from the sun
Delays escape of infrared energy
Creates moving air which distributes heat and water vapour
Provides winds over the oceans, creating water currents
Creates pressure which allows liquid to exist
Source of gases for human exploitation
What is the composition of the atmosphere?
Nitrogen- 78%
Oxygen- 21%
Carbon dioxide- 0.04%
(Combined) rare gases- 1%
Ozone- 0.000007%
Dynamic equilibrium in the atmosphere
Natural processes are in a state of balance
DE in the atmosphere maintains the composition so that it only changes over very long timescales
Photosynthesis and aerobic respiration are particularly important processes, roughly balancing eachother
However, the rates at which they occur varies over different timescales so the concentration of each gas fluctuates around a mean concentration
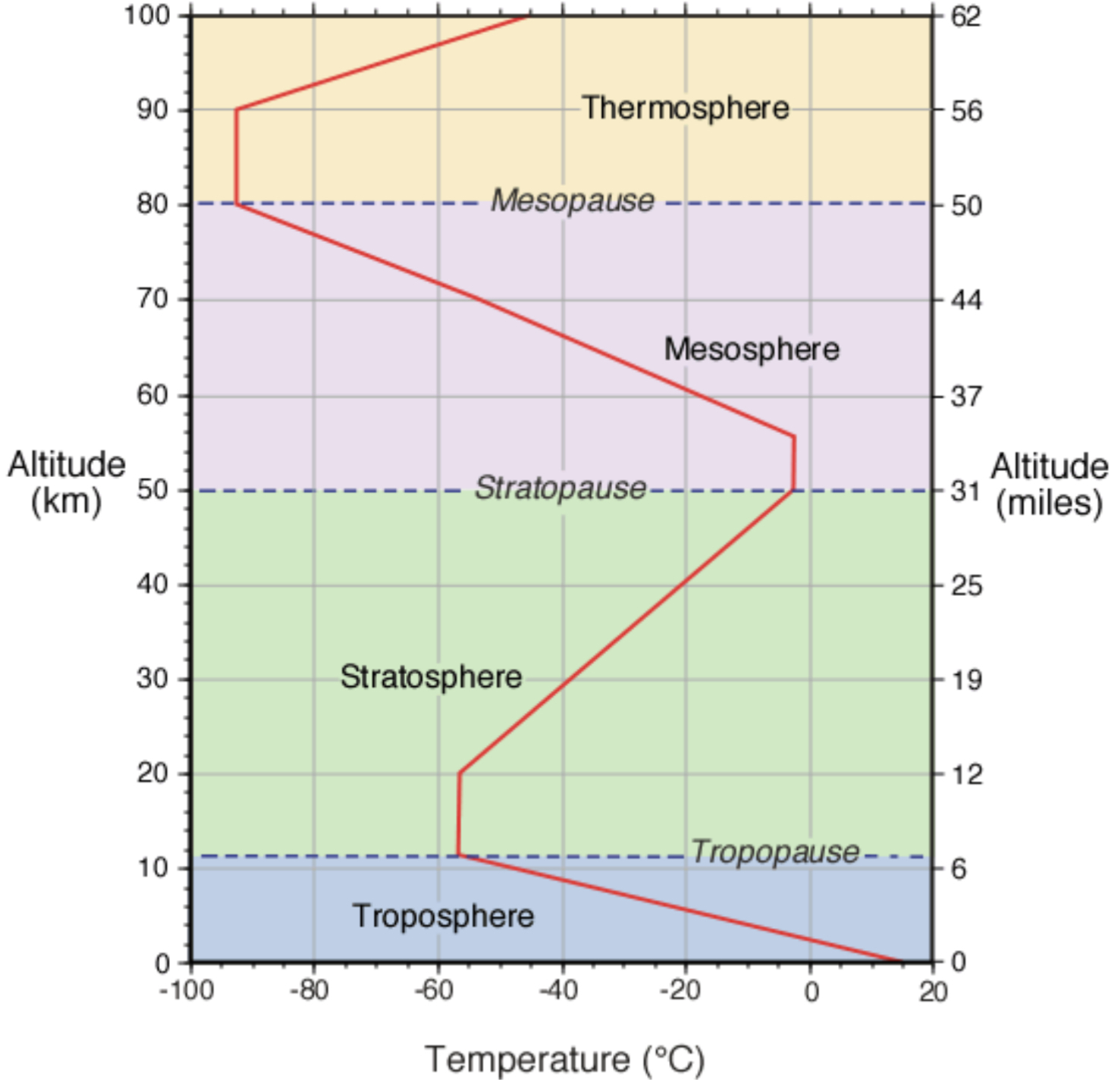
Layers in the atmosphere
TRuST METH
Troposphere
Stratosphere
Mesosphere
Thermosphere
A change in temperature with distance is called a temperature gradient.
The atmosphere is divided into layers based on:
Change in atmospheric pressure
Change in temperature
Chemical composition
Atmospheric pressure
Controls the ease with which water molecules can evaporate and escape from the water surface
If atmospheric pressure was much lower there would be no liquid water on Earth
Pressure decreases with altitude
Changes with altitude
Troposphere:
Temperature declines with increasing altitude in the troposphere due to heating by IR radiation from the ground
Also contains much more water vapour than other layers, and lots of dust particles
Stratosphere:
Temperature increases with altitude
More ozone found in the stratosphere where UV light interacts with oxygen
Suns UV heats the stratosphere
Mesosphere:
Temperature decreases with altitude due to fewer air particles and radiative emissions such as CO2
Thermosphere:
Temperature increase with altitude due to solar activity, our particles are not as well mixed in this layer
Stratospheric ozone layer
Protects us from incoming UV radiation
Ozone molecules which absorb UV later re-radiate energy as heat, warming the stratosphere
Doesn’t contribute to global warming and climate change
Tropospheric ozone
Ground level (tropospheric) ozone is created by chemical reactions between oxides of nitrogen and volatile organic compounds
The combination of these chemicals in the presence of sunlight form ozone
Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming
Graph of temperature of different altitudes showing layers
How does the atmosphere support life?
Gases for natural processes
Gases for human exploitation
Transport of water vapour
Absorption of EM radiation from the sun
Delaying escape of IR energy
Heat distribution
Ocean currents
1) Gases from natural processes
Nitrogen > DNA and protein synthesis
Carbon dioxide> photosynthesis and natural greenhouse effect
Oxygen> aerobic respiration
Water> biological solvent
All carbohydrates, lipids and proteins contain C,H,O and N
2) Gases for human exploitation
Humans extract a variety of industrially important gases
Nitrogen, oxygen, carbon dioxide
Also and inert gases such as argon, neon, krypton (they wont undergo chemical reactions with many materials)
Helium> balloons, blimps, coding in high tech manufacturing
CO2> fire extinguisher
Nitrogen> fertiliser manufacturing
Ozone> water sterilisation, treatment, purification
Oxygen> steel, iron purification, breathing gases, cutting and welding, rocket fuel, sewage treatment
3) Transport of water vapour
Winds transport water vapour to areas that would otherwise get little or no precipitation
4) Absorption of electromagnetic radiation from the sun
Lots of UV light that passes through upper atmosphere is prevented from reaching earth’s surface by the various forms of oxygen present in the stratosphere
O, O2 and O3> monoatomic, diatomic, triatomic
The 3 form is dispersed layer in the stratosphere known and the ozone layer
This absorbs / utilises UV light, producing a dynamic equilibrium of chemical reactions which form and destroy ozone
5) Delaying escape of infrared energy
Most incoming visible light is absorbed, converted to heat and re-emitted as IR energy
Naturally occurring atmospheric gases absorb this infrared energy, convert it to heat and increase the temperature of the atmosphere
This raises earths temperature in 2 ways:
Warm atmosphere emits infrared energy which is absorbed by earths surface
Warm atmosphere reduces heat loss by conduction from land and oceans
6) Heat distribution
Most energy that is absorbed from the sun is absorbed by the surface in tropical regions
Warm surface heats atmosphere above, this heat is distributed to higher latitudes by warm winds
7) Ocean currents
Winds blowing over the oceans create currents that distribute heat by carrying warm water from tropical areas to higher latitudes
e.g. North Atlantic Conveyor
These currents can also distribute dissolved nutrients