D103 Apoptosis (ALS 26, Video 39)
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
apoptosis
regulated form of cell death that occurs during development in homeostasis of organs
mass turnover in healthy human body
cotnrolled cell death for homeostasis
characteristics of apoptotic cell death
mild convolution; chromatin compaction and margination; condensation of cytoplasm
breakup of nuclear envelope; nuclear fragmentation; blebbing; cell fragmentation
phagocytosis
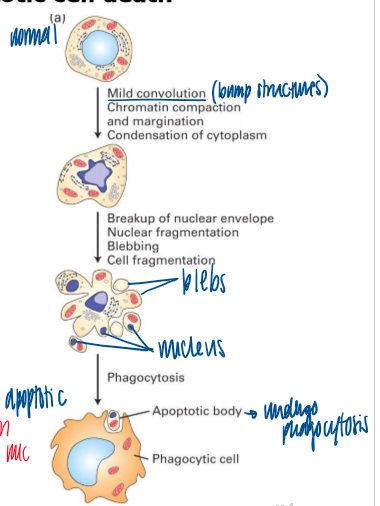
blebs
balls formed from PM of cell undergoing apoptosis
grapes
morphological characteristics of apoptotic cell death
light video-microscopy of HeLa cells undergoing apoptosis in a cell culture dish in response to an anti-cancer drug
DNA fragmentation
dna cleaves when in condensed structure via active CAD
formation of DNA ladder due to random cleavage of DNA between nucleosomes during apoptosis in mouse lymphocytes (agarose gel electrophoresis)
identification of high incidence of DNA cleavage associated with apoptosis in developing chicken limb
movement of phosohatidylserine to the exterior surface of plasma membrane
in healthy cells, PS is localized to the inner leaflet of the PM by a flippase
scramblase is off
apoptosis - PS flippase is inhibited, while scramblase is activated, so PS becomes exposed on cell surface
PS on the cell surface functions as a cellular “Eat Me” signal
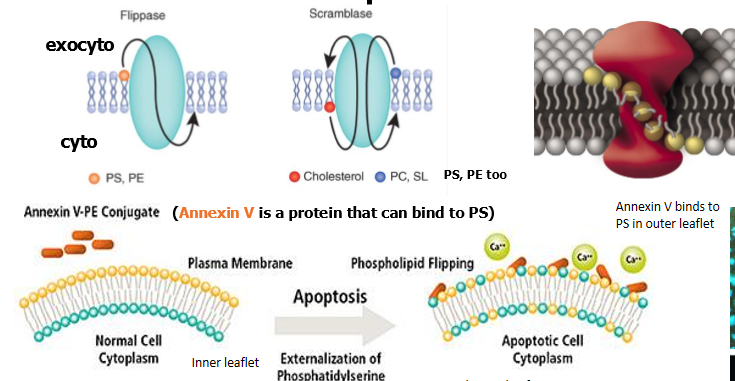
action of a flippase
entire phospholipid is switched, not just the head group
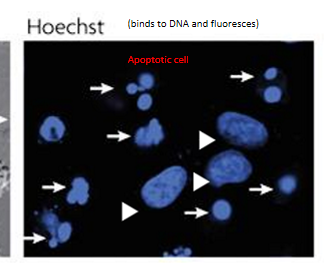
chromatin condensation
nuclear morphology of live (arrowhead) and apoptotic (arrow) cells stained with Hoechst, visualized under fluorescence microscopy
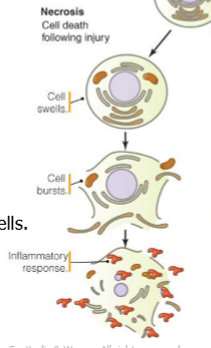
attributes of necrosis
cell swells
no chromatin condensation
no DNA laddering
cellular rupture
inflammatory response
typically affects groups of cells
attributes of apoptosis
chromatin condensation
dna laddering
cellular fragmentation without cellular rupturing
no inflammatory response
engulfment of dead cells
typically affects single cells

necrosis vs apoptosis
necrosis: swelling, rupture, inflammation
apoptosis: condensation, laddering, no cell rupture engulfment of dead cells
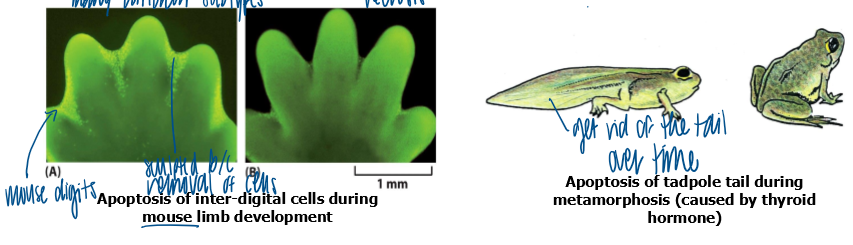
examples of function of apoptosis during animal development
sculpting
adjust cell number
deleting unwanted structures
eliminate injured or dangerous cells
disease associated with excessive apoptosis
aids
alzheimers
parkinsons
als
aplastic anemia
ischemia
disease associated with insufficient apoptosis
follicular lymphoma
breast cancer
prostate cancer
ovarian cancer
lupus
viral infections
caspases
apoptosis is effected by an intracellular family of enzymes
proteases with cysteine in the active site
cut at asp residues (c asp ases)
how are caspases synthesized
synthesized as a pro-enzyme that has very low proteolytic activity
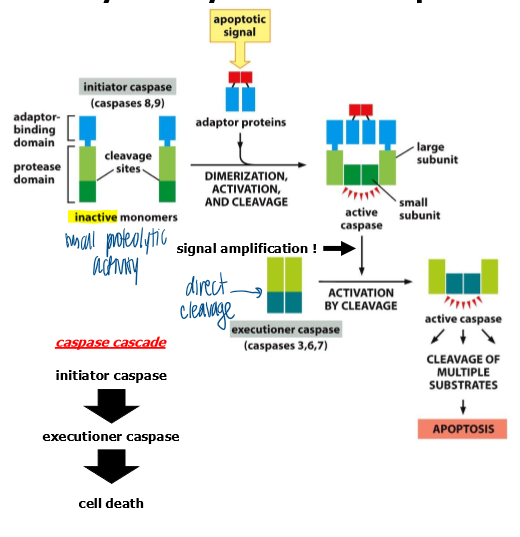
caspase cascade
when brought into proximity, initiator pro-capses cleave each other, resulting in full activation
initiator caspases activate executioner procaspases → executioner caspses disassemble the cell by cleaving essential cellular proteins
provides robust activation of cell-death processes
once fully activated = impossible for the cell to prevent cell death
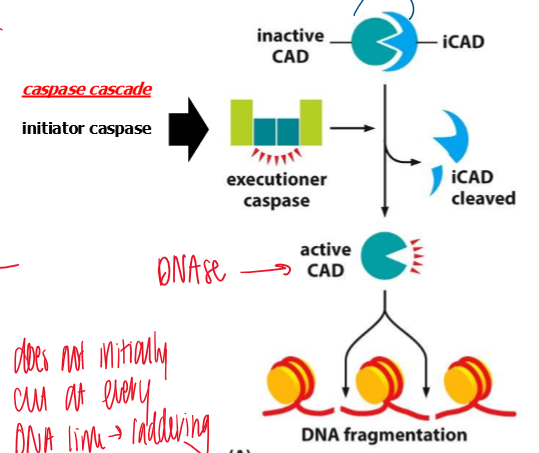
result of capase-mediated activation of DNA endonuclease during apoptosis
DNA fragmentation and DNA ladder
initiator caspase → executioner caspase activation → iCAD cleaved from the inactive CAD → active CAD → cleaves DNA
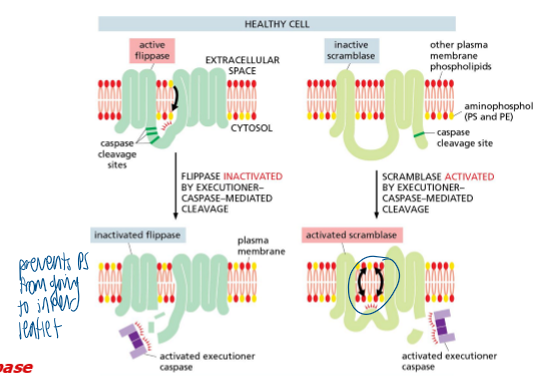
what causes accumulation of phospholipids (PS) on the surface of apoptotic cells
caspase-mediated inactivation of flippase and activation of scramblase
healthy: PS is almost exclusively localized to the inner leaflet of the PM by a flippase
apoptosis: executioner caspases inactivate flippase and activate scramblase and PS becomes exposed on cell surface
PS on the cell surface = Eat Me signal
what regulates caspase activation
fas or BCL2 pathways
both pathways involve activation of proteases (caspases) that disassemble essential cellular proteins
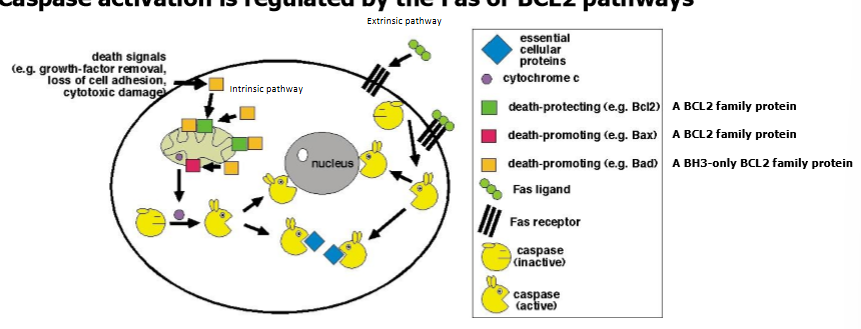
purpsoe of BCL2 and FAS protein families
control activation of caspases required for a cell to undergo apoptosis
BCL2 pathway
regulates cell death by monitoring inside the cell (cell intrinsic pathway of cell death)
involves interaction with mitochondria and release of cytochrome c from mitochondria
FAS pathway
regulates cell death by monitoring signals outside the cell (extrinsic pathway of cell death)
when activated, extrinsic FAS pathway can also activate the BCL2 cell intrinsic pathway
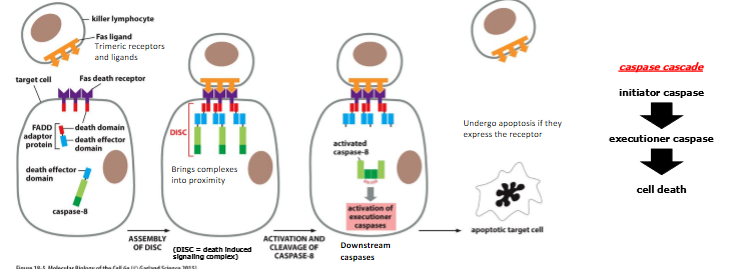
extrinsic activation of caspase cascade Via Fas-signaling
fas = death receptor located on cell surface
trimeric fas ligand binds to fas receptor and causes the receptor to trimerize
conf change in receptor recruit FADD adaptor proteins and procaspase-8, bringing the procaspases into proximity
procaspases have low intrinsic proteolytic activity
aggregation allows cleavage of procaspase-8 by each other → caspase cascade
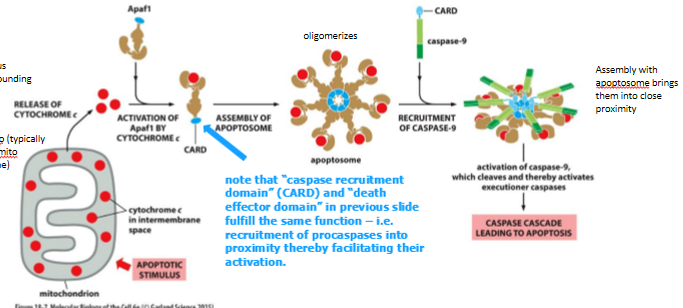
intrinsic activation of caspase cascade
cell damage or stress can trigger apoptosis
cyto-c (plus other molecules) released from mitochondria and binds to an adaptor protein (Apaf1)
Apaf1 undergoes a conformational change, which allows it to multimerize → exposes binding sites for procaspase-9 to form the apoptosome (proteolytic buzz saw of death)
cleavage and activation of procaspase-9 → caspase cascade
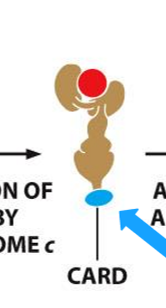
function of caspase recruitment domain and death effector domain
recruitment of procaspases into proximity thereby facilitating their activation
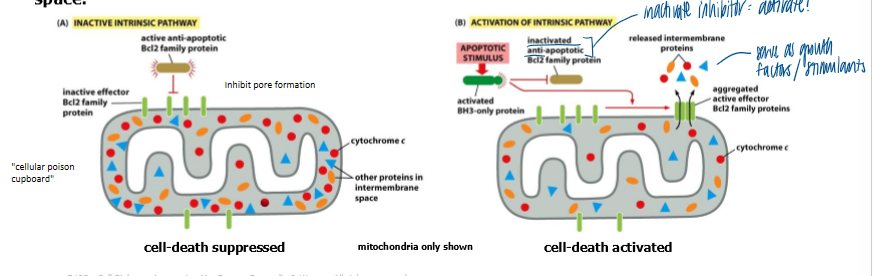
what do BCL2 family proteins control
release co cytochrome c required for activation of procaspases
regulate apoptosis by controlling release of cytochrome c and other inner membrane space proteins from mito
pro-apoptotic bcl2 proteins
promote release of cytochrome c and other proteins from the mitochondria
anti-apoptotic bcl2 proteins
try to counteract the effects of their pro-apoptotic family members by preventing release of cytochrome c and other proteins
pro and anti apoptotic bcl2 members
can bind each other directly to inhibit each other function
balance between these competing actions regulates whether a cell lives or dies
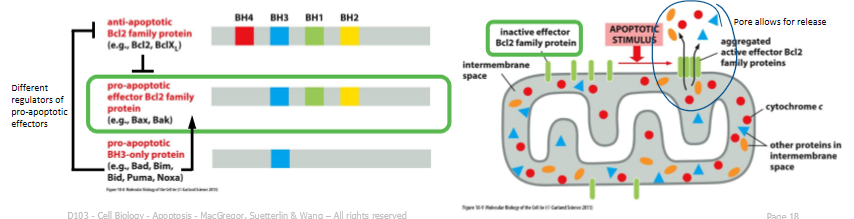
BH3 only BCL2 family proteins
pro-apoptotic and drive the release of cytochrome c during the intrinsic pathway of apoptosis
healthy cells: anti-apoptotic BCL2 family members prevent effector BCL2 family proteins from aggregating to form a channel in outer mito membrane
cell receives death signal → BH3 only domain pro-apoptotic BCL2 family members can bind to, and inhibit anti-apoptotic BCL2 family members, thereby allowing effector BCL2 family porteins to aggregate and form a channel
channels permit release of cytochrome c and other proteins from mito inter-membrane space
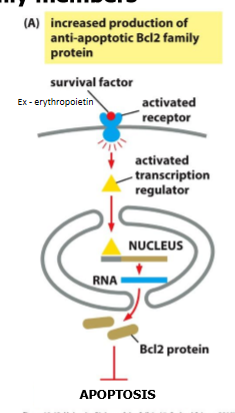
what inhibits apoptosis
extracellular survival factors alter the conc and activity of BCL2 family members
survival factors suppress apoptosis
stimlating transcription of genes encoding anti-apoptotic BCL2 family members
EpoR → STAT → BCLX
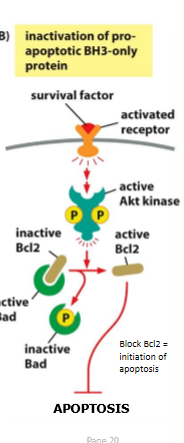
survival pathways for activation of S/T protein kinases
AKT, PKB
phosphorylate and cause temporary inactivation of BH3-inly pro-apoptotic BCL2 family members (BAD)
supply of extracellular survival factors
suppress apoptosis but limited in supply
animal cells require constant signals to promote cell survival
absence of signals = cells activate apoptosis program
ensures cells survive only when and where they are needed (ex - nerve cells using nerve growth factor)
nerve growth factors
limited and cells compete for it
ultimately only those cells that receive enough factor will survive
musical chairs
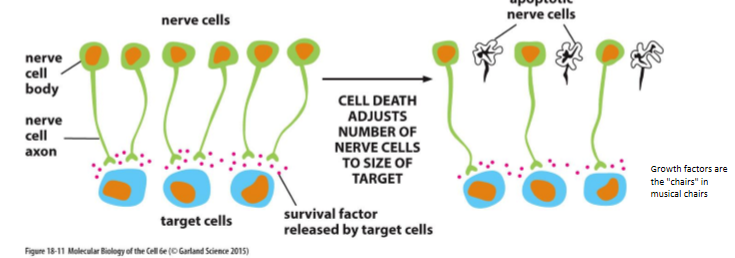
what provides extracellular survival signals
cell adhesion
cells require anchorage to survive, growth, and proliferate
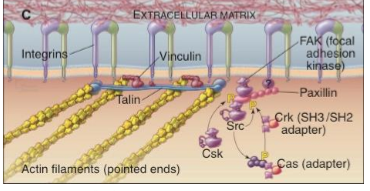
FAKs
focal adhesion kinase
associates in the cytoplasm at points of cellular contact with the substratum
signals generated at these sites stimulate cell growth, division, and survival
involve actin stress fibers and phospho-tyrosine
integrins for cell adhesion
when integrins are attached to ECM, the integrins send a signal into the cell, instructing FAK to phosphorylate tyrosines on target proteins
phosphorylation of these target proteins results in transduction of survival stimuli cell, which prevents induction of apoptosis
Which of the following is not a feature of cells undergoing apoptosis ?
A. Condensation of chromatin.
B. Ligation of DNA fragments.
C. Typically affects single cells.
D. No inflammatory response.
E. Shrinkage of cell.
B. Ligation of DNA fragments.
Which of the following genetically engineered proteins could protect cells from activating the cell-extrinsic pathway of programmed cell death when expressed in mammalian cells ?
A. APAF1 that cannot bind cytochrome c.
B. BAX that cannot oligomerize.
C. BH3-only protein that cannot interact with any BCL2 pro-survival member.
D. Pro-caspase 9 that cannot be cleaved.
E. A Fas death receptor lacking the cytoplasmic region of the protein.
E. A Fas death receptor lacking the cytoplasmic region of the protein.