🧪 Lysosomes + Vacuoles + Cytoskeleton
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Vesicle Targeting Pathways
Trans-Golgi → Endosomes
Trans-Golgi → Lysosomes
Plasma Membrane (PM) → Endosomes
Clathrin and Adaptor Proteins (AP)
AP/Clathrin-coated vesicles move from TGN to other vesicles (e.g. lysosomes, endosomes, plant vacuoles)
AP/Clathrin-coated vesicles also help form endocytic vesicles to transport vesicles from plasma membrane to endosomes or lysosomes
Summary: Depending on what the vesicles are coated with, they are sent to different areas
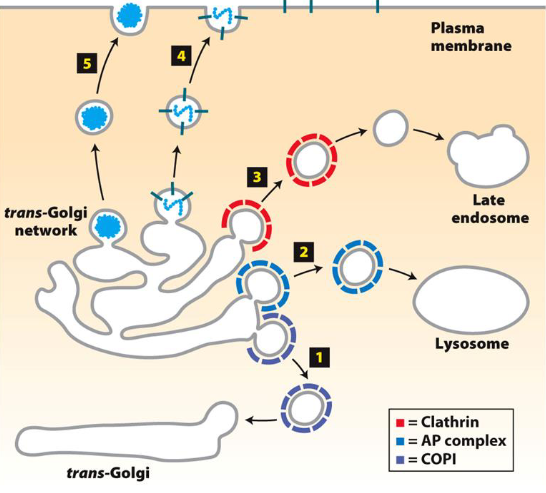
Clathrin AP (Adaptor Proteins) vs. COPs (Coat Protein Complexes)
Clathrin is a protein involved in forming vesicles, especially during endocytosis (from plasma membrane to endosomes or lysosomes)
It coats vesicles/endosomes to ensure proper trafficking and sorting of cargo
COP (COPI and COPII) proteins are involved in vesicle formation and cargo selection within Golgi apparatus and endoplasmic reticulum (ER)
Both coat proteins to help direct them to different areas
Clathrin coats are geometric, and usually for endosomes
COPI and COPII are used for Golgi-related transport (retrograde and anterograde)
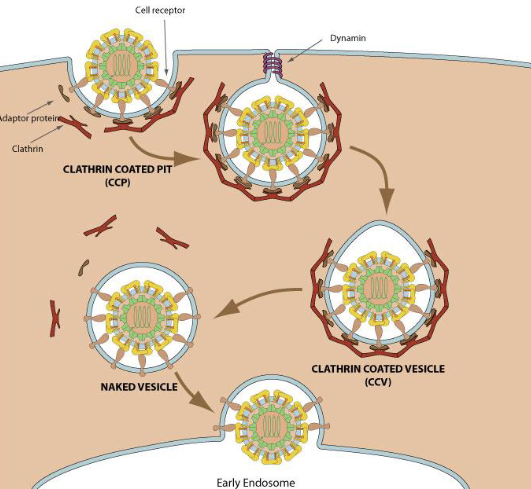
Virus Entry and Clathrin's Role
Some viruses exploit clathrin-mediated endoxytosis to enter host cells
Virus Entry Mechanism
Endocytosis: Most viruses enter host cells via clathrin-mediated endocytosis
Virus binds to cell surface receptors, triggering clathrin-coated vesicle formation
Vesicle forms, internalizes virus into cell, and moves towards early endosomes
Once inside endosome, acidification triggers the virus to uncoat and release its genetic material
Clathrin's Role
Clathrin stabilizes budding process of vesicle
Clathrin-coated vesicles facilitate internalization of viruses into host cell
The virus interacts with receptor proteins on the membrane, which then recruit clathrin to form the vesicle
After vesicle formation, dynamin helps pinch off vesicle from membrane
Outcome
Virus reaches early endosome, uncoats, and releases its genome to begin replication within the host
Clathrin plays key role in initial internalization process
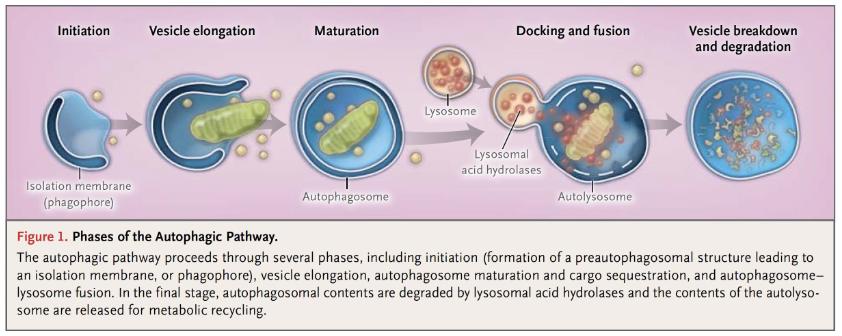
Lysosome Functions
Autophagy - Normal breakdown of unnecessary/dysfunctional organelles/components
Degradation of internalized material
a) Recycling of PM components
b) Pathogen destruction (only occurs in phagocytic cells)
Autophagy
Normal disassembly of unnecessary or dysfunctional cellular components
Involves organelle turnover
Autophagosome Formation
Isolation membrane from ER engulfs target organelles
Forms an autophagosome
Autolysosome Formation
Lysosome fuses with autophagosome to form autolysosome
Content of autolysosome is enzymatically digested and released (via exocytosis)
Autophagy Process
Autophagosome formation → Lysosome recruitment → Autolysosome → Digestion and release
Role in Cell Homeostasis
Plays important role in maintaining cell homeostasis
Degrades intracellular components and provides degradation products for recycling
Dysfunction of autophagy implicated in neurodegenerative diseases, tumorigenesis, and other conditions
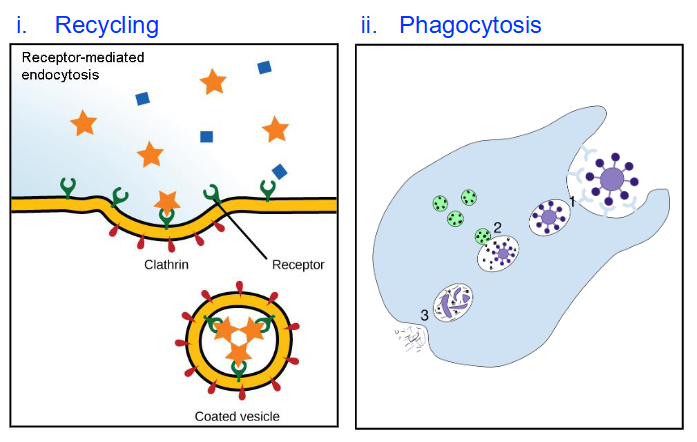
Degradation of Internalized Material
Recycling
Plasma membrane components like receptors and extracellular material are recycled
Phagocytosis (in phagocytic cells)
Pathogen (e.g., bacteria) is internalized by phagocytic cells
Pathogen-containing vesicle fuses with lysosome
Hydrolytic enzymes in lysosome degrade and kill pathogen
Debris is released outside the cell via exocytosis
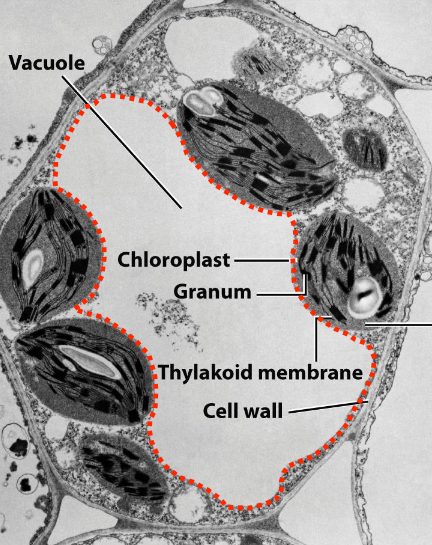
Plant Vacuole Structure
Fluid-filled, membrane-bound
Can occupy ~90% of cell volume
Surrounded by tonoplast (vacuolar membrane, - - - -)
Tonoplast contains active transport systems for ion and molecule movement
Helps in dark reactions
Vacuole Functions
Intracellular digestion
Similar to lysosomes
Slightly acidic pH (~5.0)
Contains acid hydrolases
Mechanical support & turgor pressure
Provides rigidity to plant
Supports soft tissues
Helps stretch cell wall during growth
Storage
Stores solutes, macromolecules, amino acids, sugars
Stores toxic compounds and pigments (e.g. anthocyanin)
Other roles
Regulation of cytoplasmic pH
Sequestration of toxic ions
Regulation of turgor pressure
CO₂ storage as malate
Cytoskeleton
Dynamic network of interconnected filaments and tubes
Extends through cytosol and some organelles in eukaryotes
Functions
Structural support
Spatial organization within cell
Intracellular transport
Contractility and motility
Components
Microtubules
Microfilaments
Intermediate filaments
Functions of the Cytoskeleton
Vesicle transport
Centrosome releases tubules to form cytoskeleton
Cytoskeleton forms tracks for motor proteins like kinesin and dynein
Vesicles move along microtubules to reach specific destinations (e.g. Golgi, plasma membrane)
Extension of neurites (Axons/Dendrites)
Actin filaments and microtubules support growth of neurites (axons or dendrites)
Important for forming neural connections during development
Cell division
Microtubules form mitotic spindles to separate chromosomes
Actin filaments form contractile ring for cytokinesis (splitting cytoplasm)
Cilium or flagellum
Made of microtubule-based structures
Dynein arms slide microtubules to produce bending movement
Used for motility (e.g. sperm flagellum) or fluid movement across cell surfaces (e.g. respiratory tract)
Shortening of tubules allows for movement
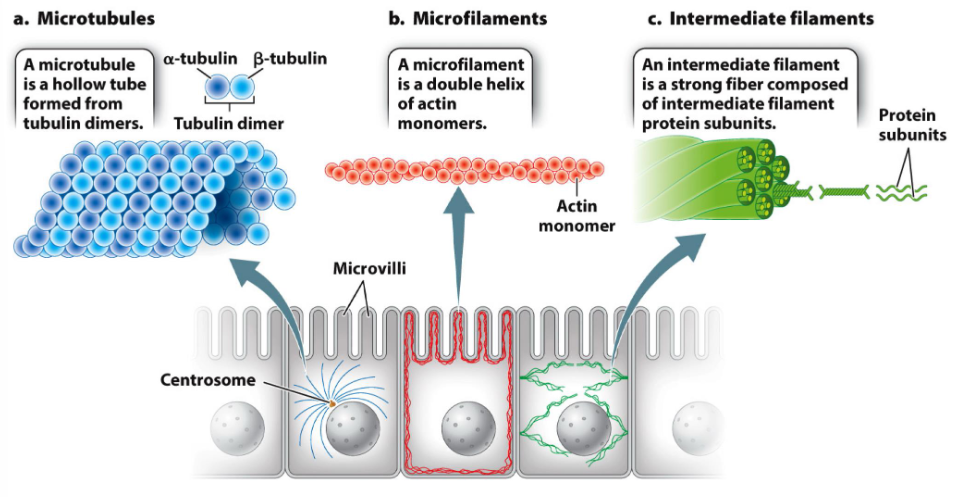
Components of Cytoskeleton
Microfilaments
7-9 nm
Double helix of actin (protein) monomers
Intermediate filaments
10 nm
Strong fiber made up of various kinds of proteins
Microtubules
25 nm
Hollow tube made up of alpha- and beta-tubulin (called dimers when they combine, also protein)
Microtubules (MT)
Largest cytoskeletal element (25 nm diameter)
Polymer of two tubulin monomers: α-tubulin and β-tubulin
Form hollow tubes made of repeating α-β dimers
Types of Microtubules
Axonemal MT
Highly organized and stable
Found in motile structures like cilia and flagella
Involved in cell movement
Cytoplasmic MT
Loosely organized, very dynamic
Found in cytosol
Involved in vesicle transport, cell shape, spindle formation during division
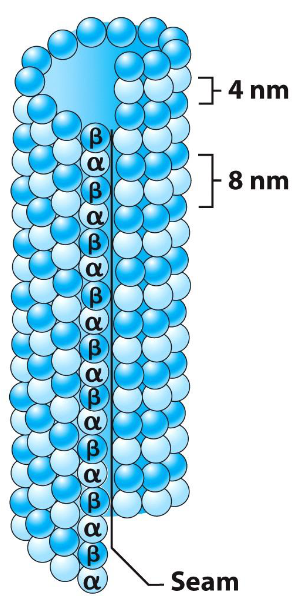
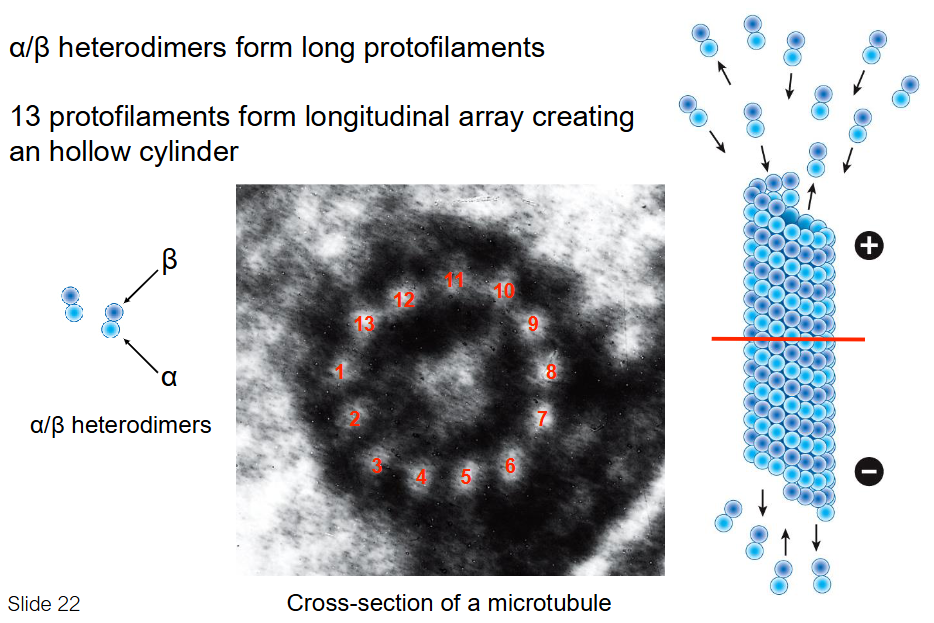
Microtubule Structure
Made of α/β-tubulin heterodimers
Heterodimers form long protofilaments
13 protofilaments align in parallel
Form a hollow cylindrical polymer
Cylinder gives rigidity and polarity to microtubule structure
Heterodimers aligned in same direction (head to tail) → creates structural polarity
Microtubules have a fast-growing + end and slow-growing - end
Polarity is important for MT growth and direction of transport along MT
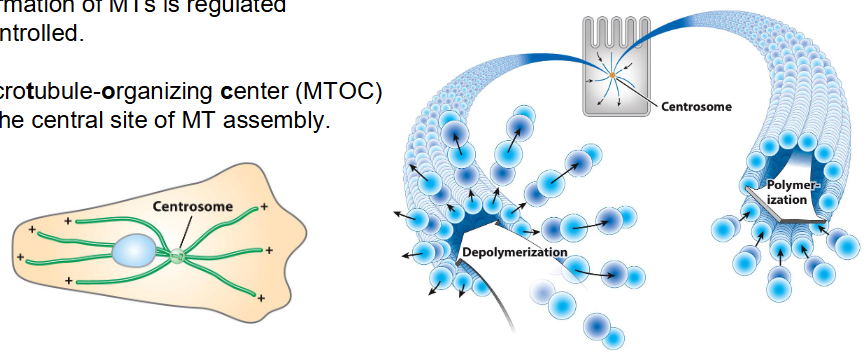
Microtubule Assembly and Disassembly
Dynamic instability → MTs rapidly grow and shrink
Half-life of most MTs in vivo is just minutes
Shrinkage at + end can happen quickly → Called catastrophe
MT formation is regulated
Microtubule-organizing center (MTOC) = Main site of MT assembly
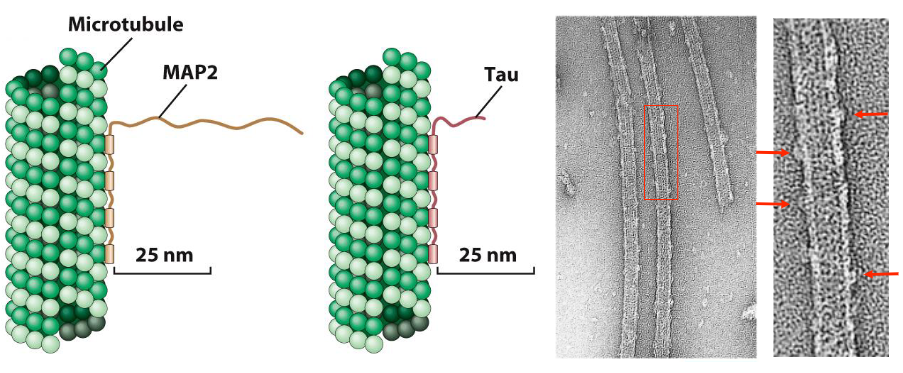
Microtubule-Associated Proteins (MAPs)
Bind to microtubules (MTs)
Modulate MT assembly and function
Mediate interactions with vesicles, organelles, other structures
Can stabilize MTs or stimulate their assembly
Tau is primarily found in axons
MAP2 is primarily found in dendrites
Classes of Microtubule-Associated Proteins (MAPs)
1. Non-Motor MAPs
Control MT organization in cytosol
Example: Tau protein in neurons
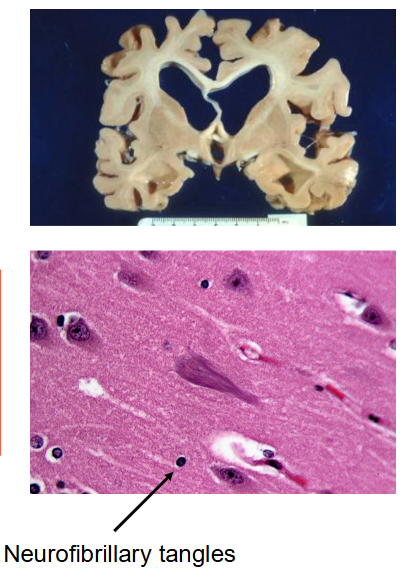
Defective Tau → neurofibrillary tangles → linked to Alzheimer’s disease
2. Motor MAPs
Two main types: Kinesin and dynein
Use ATP to generate force
Move material along MTs
Generate sliding force between MTs
Summary
Exocytosis
Secretory vesicles deliver contents outside cell
Path: ER → vesicles → Golgi → vesicles → PM
Endocytosis
Vesicles bring contents into cell
Path: Vesicles (endosomes) → Lysosomes
Autophagy
Lysosomes recycle "used" organelles
Phagocytosis
Capture and destruction of pathogens (e.g. bacteria)
Vacuoles
Plant organelles that store compounds and provide structural support via turgor pressure
Cytoskeleton
Main structural components: Microtubules, Intermediate filaments, and Microfilaments
Microtubules (MT)
Polymers of α and β tubulin
Provide structural support and intracellular tracks (in animals)