Biology Cell Membrane Quiz
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Semi-permeable: its phopholipid bilayer acts as a barrier that controls the passage of certain substances between the intracellular and extracellular environments.
Unitary: All biological membranes obey the same basic structure
Trilaminar:Its a 3-layer structure formed by phospholipids
Asymmetric: The outer and inner sides of the phospholipid bilayer have different compositions of lipids, proteins, and carbohydrates
Functions: Cell integrity
Cell Membrane
Cell Integrity
Seperates the intracellular environment from the extracellular environment
Connections to the cytoskeleton
Exchange surface of: energy, substances, and information
Cellular Recognition
Membrane Functions
Its mosaic because it's made of a variety of different molecules like phospholipids, proteins, cholesterol, and carbohydrates and “fluid” refers to the constant movement of its components, especially proteins and phospholipids.
This movement allows the membrane to be flexible and plastic, not rigid and static.
Fluid Mosaic Model
Phospholipids: are amphipathic(hydrophilic head and two hydrophobic tails) molecules that form the phospholipid bilayer of all biological membranes.
Cholesterol(animal cells): is amphipathic and helps regulate its fluidity, preventing the membrane from becoming too rigid at low temperatures or too fluid at high temperatures
Lipids of the cell membrane:
Located on the external face and can be covalently bonded with proteins(glycoproteins) or lipids(glycolipids).
These two constitute the glycocalyx which exists in some bacteria and the epithelial animal cells.
Carbohydrates of the cell membrane
Permanently embedded within the membrane.
Are amphipathic(their hydrophobic regions gain stability alongside the phospholipids’ tails and their hydrophilic regions are exposed to water.
Some can be covalently bound to lipids
Many are transmembrane proteins which cross the membrane from one side to another.
Integral Proteins
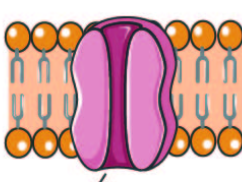
They bind to the hydrophilic regions of transmembrane proteins and phospholipids, through weak bonds(like hydrogen and ionic). These weak bonds allow them to attach and detach.
Peripheral Proteins
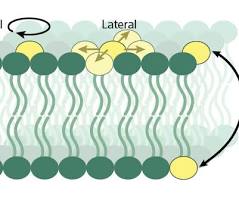
Lateral: moves sideways within the same membrane layer
Rotational: spins around its own axis
Flip-Flop(rare): moves between membrane layers.
Types of phospholipid movement
Adhesion to other cells(in animals)
Anchorage: allows tissue cohesion in animal cells; others are linked to the cytoskeleton, which maintains the cell’s shape.
Transport: substances across the membrane as channels or carriers
Enzymatic: catalyze chemical reactions
Receptors for chemical signals from outside the cells
Various protein functions
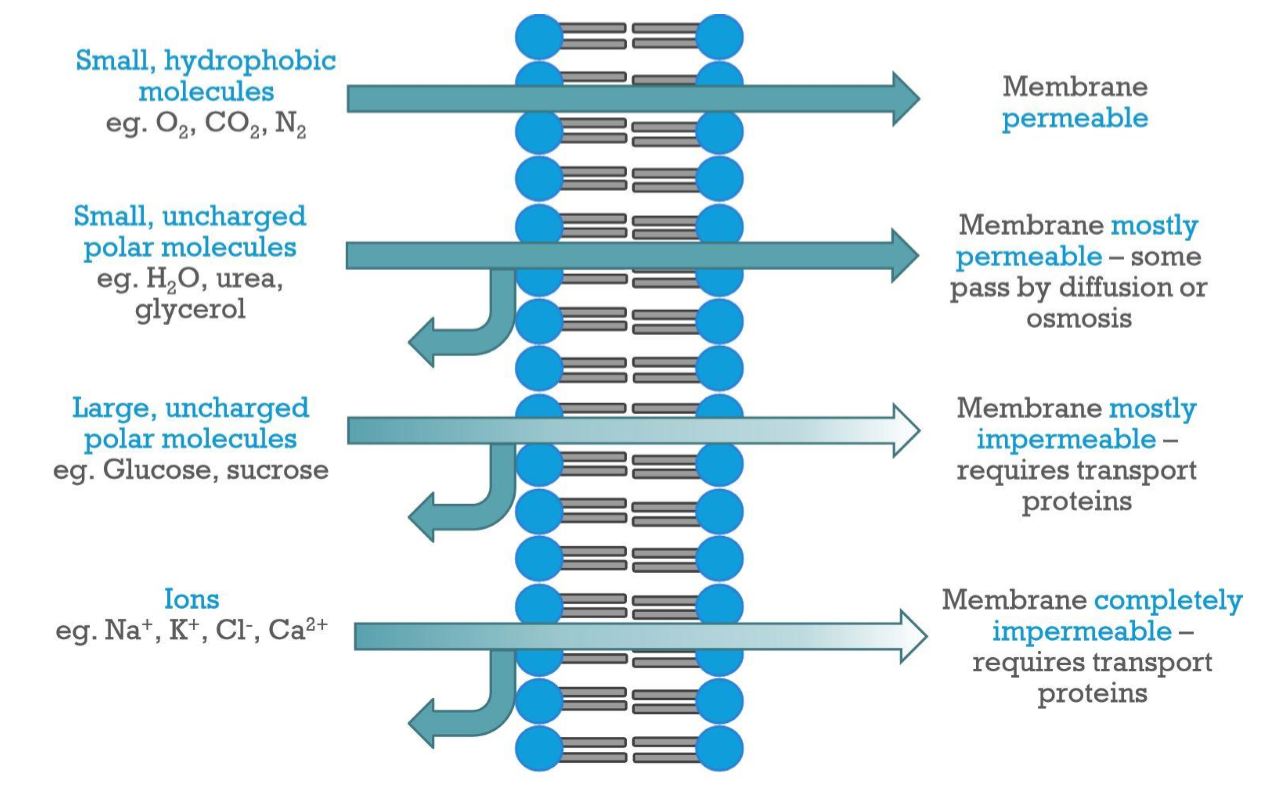
High: small, apolar molecules like oxygen and carbon dioxide can easily diffuse through the phospholipid bilayer
Mostly permeable: Small, uncharged polar molecules like water cross over more slowly
Mostly or completely impermeable: ions and large polar molecules like glucose require transport proteins
Membrane Permeability
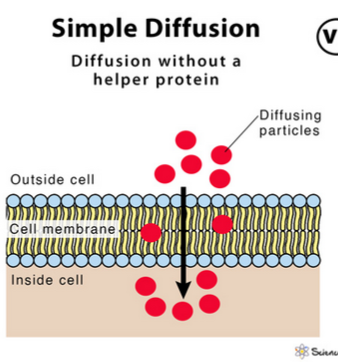
A type of passive transport where the particles of a solute move through the cell membrane from an area of high concentration to an area of less concentration, down the concentration gradient, and requires no energy. This process continues until equilibrium is reached.
Its transport rate is directly proportional to the concentration difference and has no limit.
Substances transported:
small, nonpolar molecules, or lipid-soluble molecules
Simple diffusion
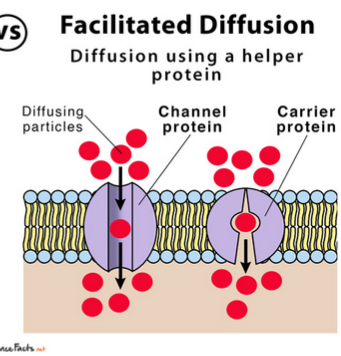
Passive transport through intrinsic proteins(channel proteins or permeases), down the concentration gradient without requiring energy use.
When all proteins are saturated with the substance, the transport rate reaches its maximum.
Substances transported:
polar molecules, ions, and larger molecules like glucose and amino acids
Facilitated Diffusion
A carrier protein that binds the molecules to be transported and undergoes a conformational change to move them across the cell membrane without using energy.
Permeases
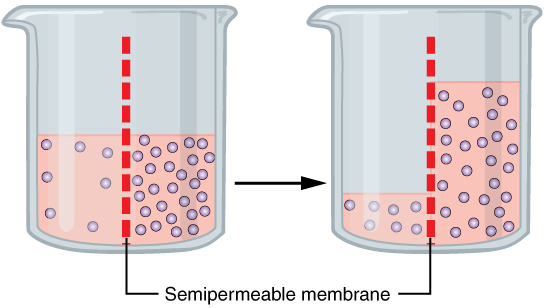
The movement of water through a semipermeable membrane from an area of lower solute concentration(hypotonic) to an area of higher solute concentration(hypertonic), until equilibrium is reached.
Cells use aquaporins, special protein channels, to move water more efficiently during osmosis.
Osmosis
A solution that has the same solute concentration as the inside of a cell, which means there is no net movement of water across the cell membrane. The cell remains unchanged in size, neither swelling nor shrinking.
Example: saline(0.9% NaCl), it’s salt concentration nearly the same as human blood.
Isotonic solution
a measure of the free energy of water per unit volume
Water potential
the pressure needed to prevent the movement of water by osmosis
Osmotic pressure
Lower solute concentration
Higher water potential
Lower osmotic pressure
Example: fresh water
Hypotonic solution
Higher solute concentration
Lower water potential
Higher osmotic pressure
Example: salt water
Hypertonic solution
Within a hypertonic environment, water leaves the cell, causing it to lose volume. (Plasmolysis)
Plasmolyzed Cell
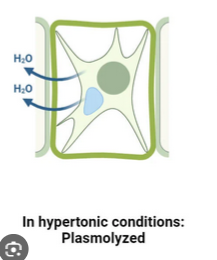
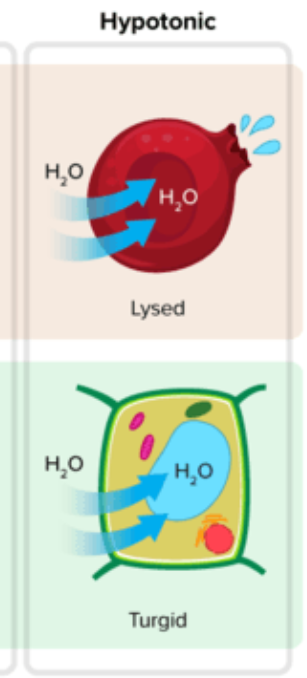
In a hypotonic environment, water enters the cells, causing it to increase in volume.
In animal cells: Cell lysis(rupture) may occur(hemolysis in red blood cells)
In plant cells: The increase in volume causes the vacuole to swell and the cell membrane to push on the cell wall, generating turgor pressure. But, the cell wall resists, preventing the cell from bursting.
Turgid cell
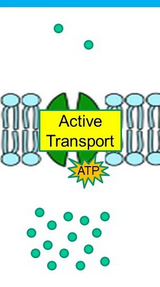
Requires energy(ATP) to move substances against the concentration gradient using specific transport proteins(protein-mediated)
Functions: incorporates necessary substances to the cell, expels cell waste products, and helps maintain homeostasis
Active Transport
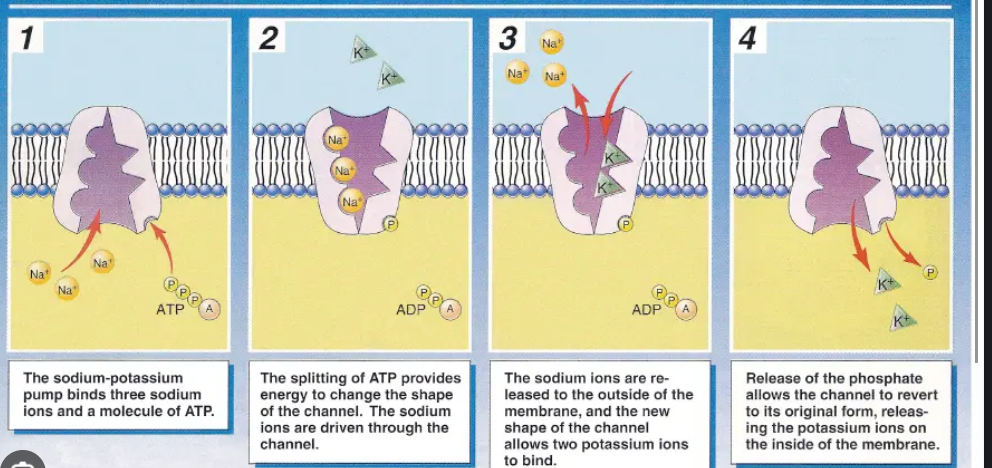
Is an ATPase transmembrane protein that transports sodium ions out of the cell and potassium ions into the cell.
Process:
3 intracellular sodium ions bind to the pump, triggering the pump to hydrolyze ATP, transferring a phosphate group to the protein(phosphorylation). This phosphorylation changes the pump’s shape and causes the pump to open towards the extracellular environment, releasing the sodium ions out of the cell. Then, 2 extracellular potassium ions bind to the pump causing the phosphate group to be released(dephosphorylation). This causes the pump to revert back to being open towards the intracellular environment, so the potassium ions are released into the cell. The pump is then ready to start the cycle again.
Sodium-Potassium Pump
Osmoregulation is crucial for homeostasis because it maintains the balance of water and dissolved solutes in an organism's body fluids, which is essential for preventing cells from swelling or shrinking due to osmosis
Importance of osmoregulation in homeostasis
A form of active transport where the cell membrane invaginates (folds inward) to take in substances(molecules, organic particles, or bacteria), creating a pocket that pinches off to form an intracellular vesicle, bringing the substances into the cell.
Endocytosis
Vesicles(phagosomes) are formed by the emissions of cytoplasmic extensions(pseudopods) that surround and engulf solid particles, such as food or bacteria, to be endocytosed.
Phagocytosis(cell eating)
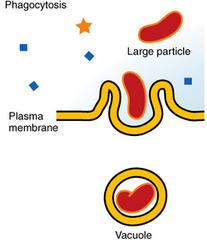
The cell membrane folds inward, engulfing extracellular fluid and dissolved solutes, and then pinches off to form an intracellular vesicle.
Pinocytosis(cell drinking)
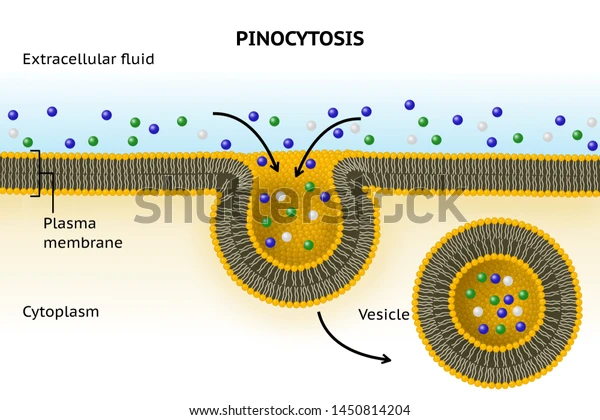
Extracellular specific macromolecules bind to receptor proteins in the membrane. This binding triggers the invagination of the membrane, assisted by cytoplasmic proteins to form a coated vesicle that pinches off from the membrane, bringing the macromolecules into the cell.
Receptor-mediated endocytosis
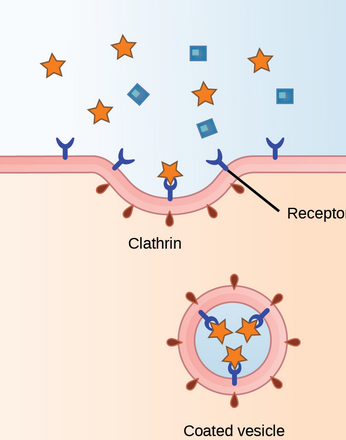
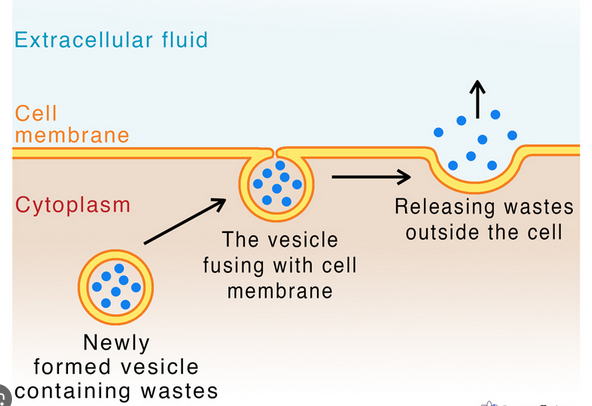
A vesicle from the cell containing substances to be released, such as waste products, toxins, hormones, or neurotransmitters, moves to the plasma membrane. It then fuses with the plasma membrane, releasing its contents into the extracellular environment.
Exocytosis