OChem
1/326
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
327 Terms
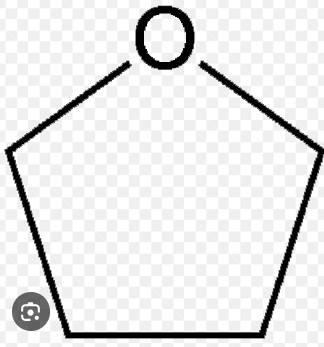
draw tetrahydrofauran
what things do we look for to find units of unsaturation
a ring struture is one degree of unsaturation
a pi bond is one degree of unsaturation
basically each missing electron pair is one degree of unsaturation
draw a ketone, what is its suffix
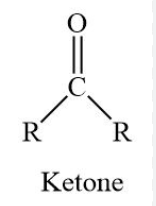

suffix is -one
draw an aldehyde, what is its suffix
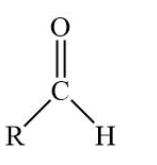

suffix is -al
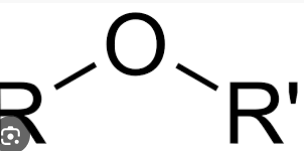
draw an ether, what is its suffix
suffix is -oxy
draw carboxylic acid, what is its suffix
suffix is -oic acid
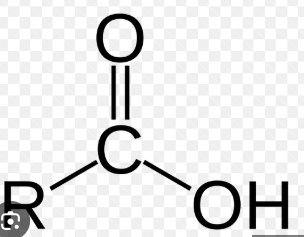
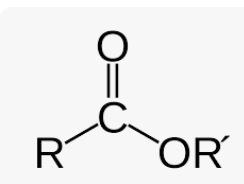
draw an ester, what is its suffix
suffix is -oate
benchmarks to memorize for nmr signal shifts
tms- 0- baseline
alkanes- 1-2ppm
halides- 2.5-4.5
aromatics- more unsat so its lower from 6-9 ppm
aldehyde- 9-10 ppm
carboxylic acid- 10-12 ppm
what is downshifting in nmr
when either being close to an electronegative atom, or an unsaturatged bond, causes deshieldijng of the electrons which causes the spectra to shift to the left of the graph/towards a higher ppm
the closer it is to this atom, the greater the shift is/the farther left it moves
on an nmr graph, is 0ppm on the right or the left
its on the right
what is a signal in nmr
a signal is produced by a unique hydrogen- we look at symmetry to determine how many uniquue hydrogen signals there will be on an nmr graph
what are splits on an nmr graph
n (adjacent hydrogens)+1
what is integration on an nmr graph
how tall each pf the lines is. it shows the abundance of each hydrogen. for example, if you have 6 of one type of unique hydrogen, and 2 of another, the one with 6 will have the tallest lines
benchmark for alkenes on an nmr graph
just left of alkanes due to higher degree of unsaturation
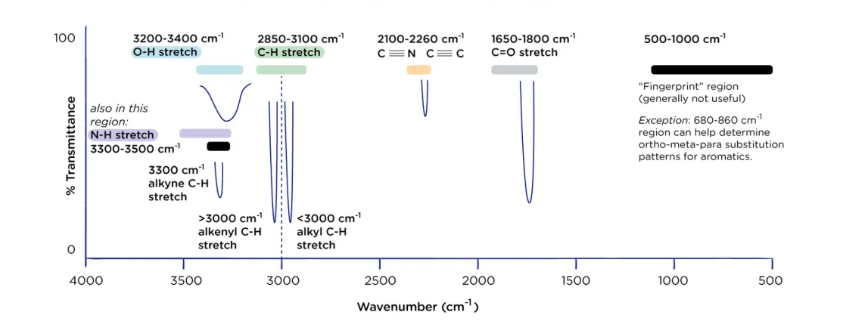
benchmarks to memorize for IR signals
33-3500- NH
32-3400- OH
3300- alkyne
alkenyl- above 3300
2850-3100- CH
under 3000- alkyl CH
2100-2260- C triple bonded with C or N
1650-1800- C=O
500-1000- fingerprint region- generally not useful except 680-860 helps determine ortho-meta-para substitution for aromatics
what is alkenyl
alkenyl- ie. vinyl/ethenyl- alkene with hydrogen removed
what is an alkyl
alkane missing one hydrogen
ie. methyl, ethyl, propyl
give benchmarks for CNMR values
0-50 ppm for alkanes, 50-100 ppm for carbons with double/triple bonds, 100-160 ppm for aromatic/alkene carbons, and >160 ppm for carbonyl carbons (aldehydes, ketones, carboxylic acids
if a CNMR graph shows a doublet, does this mean the carbon has one H attached to it, or one other C attached to it
it means it has one H attached to it- whether its a HNMR or a CNMR we always look at the number of hydrogens attached to the atom of interest to det. splitting patterns
in a assymetric molecule, the number of peaks in the CNMR graph corresponds to
the number of carbons present in the molecule
what is the J value in NMR
in an NMR spectrum, the J-value, or coupling constant, represents the separation (in Hertz, Hz) between the adjacent sub-peaks within a split signal (a multiplet) and indicates the degree of spin-spin coupling between neighboring atomic nuclei.
what is peak intwgration in NMR
it is the area under a given peak on the graph- it is equal to the number of equilivant protons giving rise to the peak
ie. a peak caused by a single proton has a area 1/3 the size of a peak caused by a methyl group
what causes nmr signal splitting
spin spin coupling- The source of signal splitting is a phenomenon called spin-spin coupling, a term that describes the magnetic interactions between neighboring, non-equivalent NMR-active nuclei. In our 1,1,2 trichloromethane example, the Ha and Hb protons are spin-coupled to each other. Here's how it works, looking first at the Ha signal: in addition to being shielded by nearby valence electrons, each of the Ha protons is also influenced by the small magnetic field generated by Hb next door (remember, each spinning proton is like a tiny magnet). The magnetic moment of Hb will be aligned with B0 in (slightly more than) half of the molecules in the sample, while in the remaining half of the molecules it will be opposed to B0. The Beff ‘felt’ by Ha is a slightly weaker if Hb is aligned against B0, or slightly stronger if Hb is aligned with B0. In other words, in half of the molecules Ha is shielded by Hb (thus the NMR signal is shifted slightly upfield) and in the other half Ha is deshielded by Hb(and the NMR signal shifted slightly downfield). What would otherwise be a single Ha peak has been split into two sub-peaks (a doublet), one upfield and one downfield of the original signal. These ideas an be illustrated by a splitting diagram, as shown below.
if 2 spins are aligned it…, what about if they are unaligned
deshields the H, while if they are unaligned it shields the H (deshielding shifts downfield, shielding shifts upfield)
vicinal vs geminal hydrogen
Vicinal hydrogens are located on adjacent carbon atoms, while geminal hydrogens are located on the same carbon atom.
what is j coupling
when the spin of one proton infuences the spin of its neighbor via the bonding of electrons
the j coupling constant measures the stregth of the spin spin interaction between bonding electrons between 2 nuceli
Coupling is reciprocal: the energy splitting of proton A caused by proton B is exactly the same as the splitting of proton B caused by proton A.
So if HA_AA and HB_BB are 3 bonds apart (vicinal), you’ll have one value JABJ_{AB}JAB.
HA_AA appears as an n+1 multiplet with splitting JAB_{AB}AB
HB_BB appears as an n+1 multiplet with the same JAB_{AB}AB
t or f- only nonequilivant hydrogens can split the signal
true
2 nonequilivant adjacent protons split the signal into a
triplet
what are qualities in a good nmr solvent
should have high purity, be inert, not peak the spectra (deuterated)
for carbons of equal hybridization, the higher the shift value in the nmr, the ___ the electroneg of the atoms bound to that carbon
greater - the higher the shift value, the more downfield it is, the more deshielded it is, the more it is bound to somehting electroneg like oxygen
what does a 3H singlet indicate
a isolated methyl
what does a 2H quartet and a 3H triplet indicate
an isolated ethyl
write the compound name for each functionality
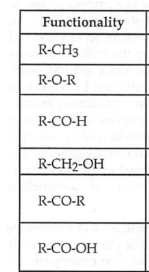
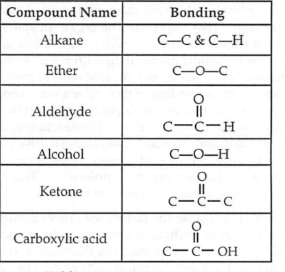
what is a ligand bond
a cooridinate covalent bond formed when electrons are donated from a ligand to a metal or ion
think lewis acid base bonds
what is special about a ligand bond
they are different from a normal covalent bond in that instead of having one electron come from each atom, both electrons come from a single molecule
how do we measure the relative electronegativity of 2 atoms
by measuring the dipole moment of the bond they form
electronegativity values and what type of bond they represent
under 1.5 diff- covalent
over 2- ionic
between 1.5 and 2- polar covalent/partially ionic
2 types of covalent bonds are
sigma- electrons between nuclei
pi- electrons above and below nuceli
between sigma and pi bonds which is stronger
sigma is stronger- more orbital overlap creates a stornger bond
the longer the bond length, the … the orbital, so d..>d..>d..
the longer the bond length, the larger the orbital, so dz>dy>dx
what is an antibond
2 orbitals which have opposite spins and lead to bond breaking when they are paired with a bonding orbital
for electron densities to overlap they have to have the same spin direction- they cant overlap if they are opposite so it creates an antibonding
for electron densities to overlap, the electron orbitals must have the …. spin direction
same
the longer the bond, the …. electron density overlaps, and the …. the bond is
the longer the bond, the less electron density overlaps and the weaker the bond is
the more substituted a bond is, the … it is since electrons become more …
the more substituted a bond is, the weaker it is since the electrons become more spread out
HONC shortcut
hydrogen froms 1 bond, oxygen forms 2, nitrogen forms 3 and carbon forms 4
define hybridization
when we relocate electron density in atomic orbitals prior to bonding to minimize repulsion between electron pairs and allow for bonding of atoms
describe the angle, shape, number of sigma bonds and electron pairs, and number of pi bonds, in each


what is special about borane
the borane atom has only 3 valence elctrons so a neutral borane cant satisfy the octet rule
how do we form a sp hybridized orbital
mixing the s orbital and the px orbital to form 2sp orbitals
if sp hybirdizatino creates a linear arrangement, does that mean everything that is sp hybridized will have a linear geometry
not necessairly- this means electron orientation will be linear, but the actial geometry of the moelcule will depend on which substitutients are attached- ie. if 2 atoms are bound to it it will have a diff arrangement than if one atom and one electron pair are present vs if it is just lone electron pairs
bond dissociation energy
the energy required to cleave a bond- helps compare bond strength
what does energy do when we break vs form bonds
energy is released to form a bond and absorbed to break a bond
how do we find enthalpy of rxn
enthalpy of rxn is like the energy in the rxn- subtract energy released toform bond-energy required to break bond
how do we know that aromatics are more stable than aliphatics
aliphatic- open carbon chain
because energy is released when the last pi bond in an aromatic ring is formed showing that aromatic structures are more stable
the greater the bond dissociation energy, the … the bond
stronger the bond is- bond dissociation energy is the amount of energy needed to break a bond- the more energy is needed, the stronger the bond is and the higher the dissociation is
columbs law
tells us the strength of an ionic bond
force between 2 charged specials equals a constant k times the charge on each ion divided by the square of the distance between the 2 charges
force (F) is given by the formula F = k (q1 q2) / r², where 'k' is Coulomb's constant, 'q1' and 'q2' are the magnitudes of the charges, and 'r' is the distance separating them
are ionic bonds stronger or weaker than covalent ones
stronger (however, since they can be solvated they are often cleaved more easily)
the columbic attraction of ions to water is comprable to…
the attraction of the ions to one another- ions are super easily solvated
5 intramolecular forces
resonance- electron density shifts through the molecule via pi bonds
inductive effect- delocalization of electrons via the sigma bonds- electroneg atoms pull density from its niehbor, which pulls from its neighbor- effect dissipates over 3-4 atoms
steric interactons- steric hindreance- any time 2 atoms are trying to be in the same place at the same time- it is repulsive and inc the closer atoms get to one anotther
aromaticity- stability generated when a molecule has 4n+2 pi electrons in overlapping ring of pi orbitals
hybridization- impacts bond angles- sp3 can have tetrahedral/109 degrees, but the more lone pairs it gets the more repulsion there is and the lower the bond angle willl be
what is a halogen
F/Cl/Br/I/As- anythng fom group 17- form super strong acidic compounds with H and are very electronegative
electroneg vs eelctropos
An electropositive element has a strong tendency to lose electrons, forming positive ions, and is typically a metal. An electronegative element has a strong tendency to attract electrons, forming negative ions, and is typically a nonmetal.
what do elecroneg atoms do to acidity
Electronegative atoms increase acidity by polarizing and weakening the hydrogen-bond (making it easier to donate a proton) and by stabilizing the resulting conjugate base's negative charge through inductive effect. Essentially, the more electronegative atom pulls electron density away, making the proton more likely to leave and the remaining negative charge on the molecule more stable.
what is a resonance hybrid
looks at all possble arrangements of pi bonds and puts them together in one sturcure- ie.
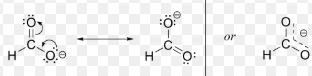
the huckel rule
a molecule is aromatic if it has 4n+2 pi lectrons, where n is a non negative integrer
what does an increased inductive effect do to the rate of a nucleophilic substitution rxn
as electrons are pulled off of the molecule,m the molecule becomes less electron rich and tus less of a nuclophile which dec the rate of rxn with an electron poor molecule
resonance vs inductive effect
resonnce- moves via pi bonds
inductive effect- moves via sigma bonds
bond angles for tetrahedral, trigonal planar and linear
tetrahedral- 109
trigonal planar- 120
linear- 180
bronsted acid
donates hydrogen
bronsted base
accepts hydrogen
is H or H+ a proton
H+ is a proton- H usually has one electron and oe proton, but if it loses the electron all it has left is a proton so we call it a proton and say its H+- protonation is when a molecule gains a H+to
to be a bronsted lowry base, a compound must have a
lone pair of elctrons it can use toform a bond with h+- because of this all bronsted lowry bases are also lewis bases
5 strong acidsd to memorize
H2SO4, HNO3,HCl, HBr, HI
as a bronsted lopwry acid becomes strong, what happens to the conjugate base and why
as the acid gets stronger, the conjugatebase gets weaker because it becomes less willing to accept protons as the conjugate acid becomes more willing to donate them
lewis acids and bases- talk quick abot ti
acid- accepts electrons- can have a protic H or an empty valence shell capable of accpting electrons- different from bronsted acic swhich has to have a H+
bases- donates eectrons- binds to any electron deficient/nucelophille, including H+- not the same as bronsted which has to bond to H+
arrhenius acid and base
acid yields hydronium and base yieldd hydroxidew
5-10-15-20 rule
the pka for a carboxylic acid is 5
pka for phenol is 10
pka for alcohol is 15
pka for proton alpha to carbonyl is 20
primary and secondary facotrs affecting acid strength
primary- depend on the bond to the acidic propton the weake the bond, the more readily it breaks and the more acidic the acid. includes…
atomic size- smaller atoms form shrrter bonds to H which are stronger- if the strength of an acid is how easily it ives up H hen the stronger the bond the wesker the acid
long bond=weaker=donates H easily=strong acid
short bond=weaker=wont donate easily=weak acid
electronegativity- seen wen we copare acids of the same row/group since they are all approx the same size- when H is bound to an atom that is more electroneg the electrons get pulled more which means H can be cleaved more easily
hybridization- compare acids when hydrogen is on the same atom- impacts bond length and distribution of electron density- as hybrid orbital gets smaler, electrons are held closer to the nucelus of the atom bound to hydrogen so the bond can be cleaved more easily- the more s character in a hybrid orbital the stronger the acid- sp is more acidic than sp2 whic is more acidic than sp3
secondary effects- resonance (delocalization of electrons in a molecule via pi bonds), the inductivde effect (delocalization of electronsvia sigma bonds), electron cloud repulsion and aromaticity- involve intramolecular forces which dictate the electron density in a molecule and thus the reactivity of the molecule
resonance and inductive effect, with resonsance beinga more significant contributor
resonance- delocalization of electrons via pi bonds- pi bonds withdraw electrons whic inc the acidicty of ti by dec the stability of the bond to H. if an adjacent atom has a lone pair and is not part of a pi bond, it is electron donating- donsting electrons will dec the acidity of the atom that donated them
inductive effect- delocalization via sigma bonds- electron density pulls toward more electronegative one which destabilizes the bond to H which inc ease with which H bond can be broken which inc acidicty- this effect is completely negligible when they are more than 4 atoms apart in the molecule
as a bond weakes, the homolytic and hterolytic bond dissociation energy…
decreases
as a bond to a protic hydrogen weeakens, the acidicty of the molecue…
inc as it becomes more likely to donate the H+ (donating H is bronstwd lowry)
with refeence to atomic size, are thiols or alcohols stronger acids
ie. thiols are stronger acids than alcohols- sulfur is a larger atom than oxygen and thus thiols are more easily deprotonated making thhem a stornger acid
remember: atopmic size is a primary effect on the strength of acids
talk about the strength of conjugate bases and atomic size
atomic size in bases: a conjugate base is more stable if electron density is spread out over more space- since larger atoms better stabilize negative chargesm they are less reactive and thus less basic (polarizability)
when will atomic size not have an imact on the strength of an acid or base
when the hydrogen is no directly boundto the atom which has a relevant size
does atomic size or electronegativity matter more when looking at primary effects n acid strength- justify youe answer with reference to the haloacids
this only works when the protic hydrogen is directly bonded to the larger atom- explains the acidity of haloacids- HI>HBr>HCl>HF- shows that size is more important than electronegativity for atoms in the same column of the periodic table
F is mroe electronegative than I, but I is larger which meand its bonds are longer which mans they are weaker which means itnholds onto H ess tightly which means it donates H more easily which means ti is a stringer acid
are alcohols or amines (ammonia with hydrogens replaced) of equal substitution more acidic
ie. alcohols are more acidic than amines of equal substitution since the electronegativity of oxygen is greater than that of nitrogen and both are of approx equal atomic size
are amides or alcohols better bases
similarly, because nitrogen is less electronegative than oxygen, it more readily donates a pair of electrons to a proton and is thus more basic (amides are super strong bases)



kiunetic vs thermodynamic product
kinetic product- formed faster due to lower activation energy
thermodynamic product- more stable and favored at equilibrium
talk about hydrogen bondig
strongest intermolecular force
5-8 kcal/mole
between lone pair of electrons and hydrogen with a pos charge- H has a pos charge when ti is bound to a small electroneg atom like N,O or F
no hydrogen bonds when H is bound to carbon (because electroneg diff is not high enough to cause partial pos on H)
is H bond stronger between alcohol or amines
hydrogen bodning is stronger in alochols than amines since oxygen is more electronegative than nitrogen and therefore alochols have an inc boiling point than amines
whenever a compound has a H bond, we know it also had a
compounds that form hydrogen bonds are also polar, so when a compound has a hydrogen bond it also has dipole-dipole interactions
polar interactions
weaker intermoelcular force than H bonds
beetween partially charged particles (1-3 kcal/mole)
neg charged sites attract pos ones, and the greater the partal charge on the site of the molecule the stronger the force betweenn opposite charges
strength inc as the dist between molecules dec
3 intermoelcularnforces from strongest to weakest
H bond, then polar interactions, them van der waals
talk about van der waals
weakest intermelcular force
considered only when no toher force is present
attraction between temporary dipoles
under 1kcal/mole
molecule weight and melting/boiling point
heavier molecules are harder to move from a lower energy state into a higher one- the more it weights, the higher the molting and boiling point
moleculkar flexibility and melting/boiling point
having a low flexibility moelcule means it may be able to pack down better and become mroe compact, which inc mslting point
having a molecule with high flexibility can inc surface area and opporutnity for intermoelcular forces like van der waals which inc boiling point
solubiity vs miscibility
solubility- the ability of a solute/sollid to dissolve into a solvent
miscibility- the ability of a liquid to dissolve into another liquid
what intermoelcular forcesd are present in a poalr and protic, polar and aprotic, and nonpolar and aprotic molecules
polar and protic- able to hydrogen bond
polar and aprotic- no hydrogen bonding, but has dipole-dipole
nonpolar and aprotic- weak intermolecular forces- this will only have van der waals
micelle
micelle- little pockets with an organic core and polar head which sticks ouut to interact with water
bond dissociation energy vs rxn enthalpy
Bond dissociation energy (BDE) refers only to the energy required to break a bond, while reaction enthalpy (ΔH) includes both bond breaking and bond forming.
are substituted carbons more or less stable
The more substituted a carbon is, the more stable it tends to be — especially in the context of alkenes, carbocations, and free radicals.
A tetrasubstituted alkene is more stable than a disubstituted or unsubstituted one due to hyperconjugation and inductive effects from neighboring alkyl groups.