CHEM 1112: Kinetics
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
- reaction rates
never possible
cant have - products forming
- sign placed infront of rate of change of reactuants in order to provide a positive rate of reaction
Reaction rate
the speed at which the chemical proceeds
independent of ∆G
amount of product formed per unit of time
always expressed as a positive #
products appear (rate is positive)
reactants disappear (rate is negative)
Rate expression
for the simple reaction A → P
rate = ∆[P] / ∆time = Pf - Pi / Tf - Ti
for complex rxn aA + bB → cC + dD (lowercase = mole ratio)
rate = -(1/a)(∆[A]/∆t) = -(1/b)(∆B/∆t) = +(1/c)(∆[C]/∆t) = +(1/d)(∆[D]/∆t)
the rate should be the same, not matter which reactant/product you use
What affects rate
concentration of reactants
temperature
presence of a catalysts
Average reaction rate
the rate at which a reaction proceeds over a time period
calculated using concentrations at the beginning and end of a time period
Instantaneous reaction rate
the rate at which a reaction is proceeding at a specific time or concentration
Zero order reaction
n = 0
rate of reaction is completely INDEPENDENT from the concentration of a reactant
integrated rate law:
[At] = -kt + [Ao]
half life:
t1/2= [Ao]/2k
1st order reaction
n = 1, [A]1
rate of reaction is DIRECTLY proportional to concentration of one reactant
integrated rate law:
ln[At] = -kt + ln[Ao]
half life:
t1/2= 0.693/k
2nd order reaction
n=2, [A]²
rate of reaction is proportional to concentration of the square root of one reactant
integrated rate law:
1/[At] = -kt + 1/[Ao]
half life:
t1/2= 1/k[Ao]
Overall order
k[A]m + [B]n
overall order= m + n
gives an understanding of how all the reactants contribute to the rate of a reaction
Integrated rate law
rate law which is integrated with respect to time to produce a concentration-time relationship
Half life
time required for the concentration of the reactant to fall to ½ of its initial value
Carbon dating
rate of decaying of carbon-14 after death may be tracked and used to date objects up to 50,000 years old
Collision theory
reactants must collide in order to react with each other
postulates:
rate of reaction is proportional to rate of collisions
molecules must be properly oriented when they collide
molecules must have sufficient Ea to react
Activation energy
Ea
minimum energy necessary to form a product during collision between reactants
appears as a PE ‘hill’ between reactants and products
only colliding particles that are properly oriented can deliver KE into PE at least as large as the Ea ‘hill’, so that products may be produced
Collision orientation
molecules must be oriented properly when they collide in order for new bonds to form
chemical reactions involve bond breaking and/or bond forming
new bonds cannot form if the appropriate orbitals cannot overlap as they form molecular orbitals
Transition state
activated complex
species at point of highest energy
exists transiently, cannot be isolated
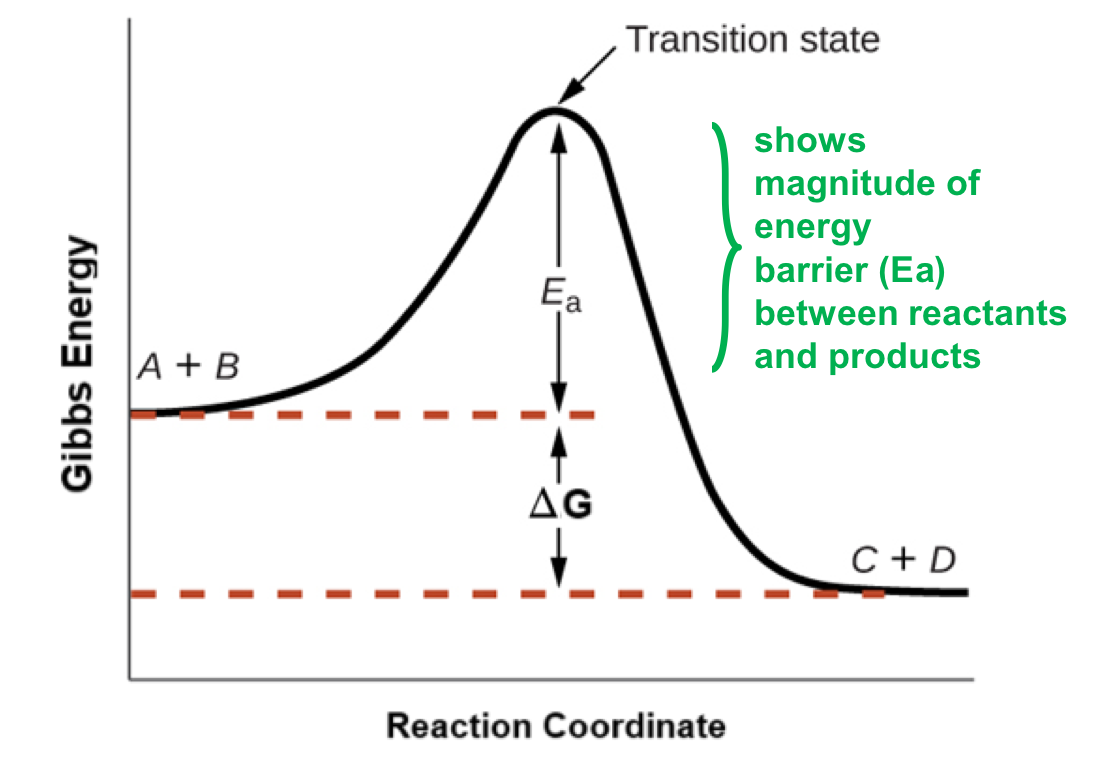
Reaction intermediate
a rxn may occur in more than one step
each step has its own activation energy barrier (Ea) and transition state (TS)
intermediate (int) shows on energy diagram as an ‘energy pit’
Arrhenius equation
k = Ae-Ea / RT
k = rate constant
Ea = activation energy
R = gas constant (given)
T = temperature (K)
A = frequency factor
used to describe temperature dependence of reaction rates
k in Arrhenius equation
dependent on temperature
higher T → higher k
at higher temperatures, more molecules have enough KE to overcome activation energy barrier, thus increasing rate constant
dependent on Ea
higher Ea → lower k
Reaction mechanism
exact molecular pathway that starting materials follow on their way to becoming products
reaction mechanism can not be determined simply by looking at the stoichiometry of the reaction
must be determined experimentally
Elementary step
each step in a multi-step reaction
Molecularity
number of molecules on the reactant side of the chemical equation for the elementary reaction
unimolecular
bimolecular
termolecular
Unimolecular
single molecule reactant
A → product
(elementary) rate law:
k[A]
Bimolecular
2 molecule reactant
A + B → product
2A → product
(elementary) rate law:
k= [A][B]
Termolecular
3 molecule reactant
A + B + C → product
2A + B → product
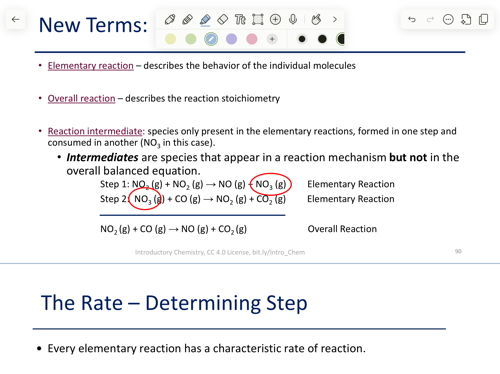
Elementary reaction
describes the behaviour of the individual molecules
Reaction intermediate
species only present in the elementary reactions, formed in one step and consumed in another
Rate determining step
each elementary reaction has a characteristic rate of reaction
rate determining step is the slowest elementary step in a mechanism, thus governing the rate of the overall chemical reaction
the rate law is related to the mechanism of the reaction
Linking mechanism and rate laws
the mechanism is one or more elementary reactions describing how the chemical reaction occurs
a satisfactory mechanism must be comprised of ‘reasonable’ elementary steps
species proposed must exist (cant be half an atom)
stoichiometry must be reasonable
sum of the individual steps in mechanism must give the overall balanced chemical equation
the reaction mechanism must be consistent with the experimental rate law
RDS and rate law
when the first step of a mechanism is RDS, the predicted rate law for the overall reaction is the rate law for that first step
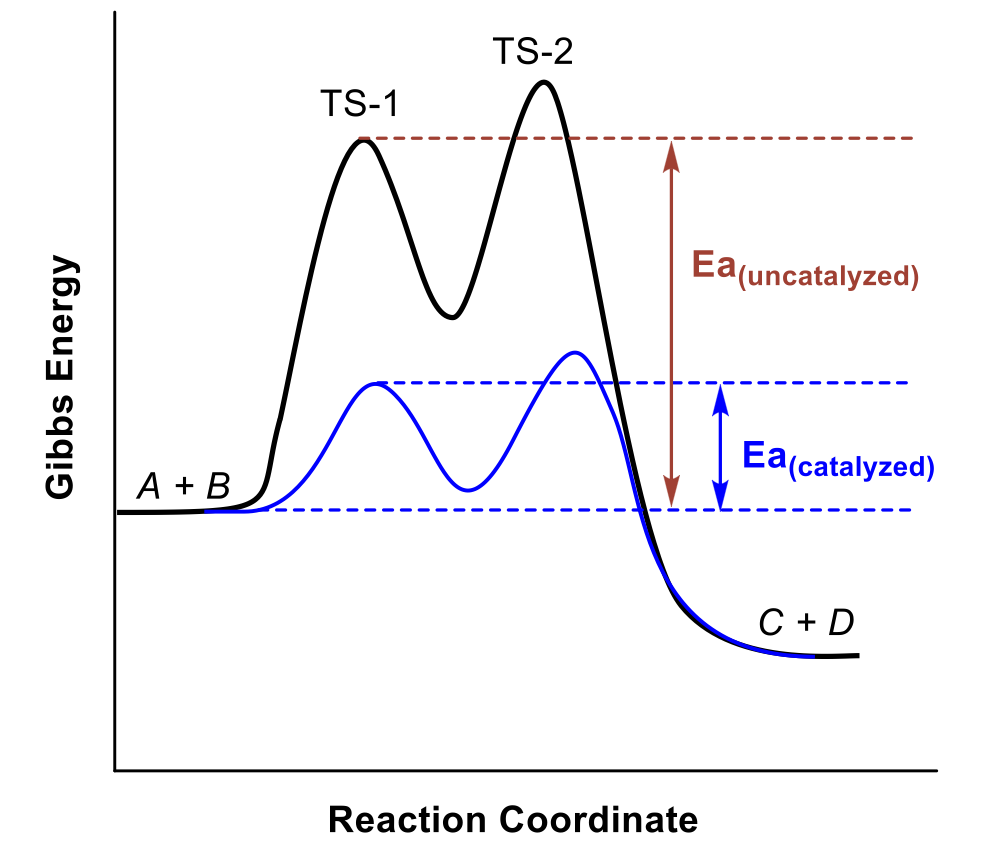
Catalyst
a substance that increases the rate of a chemical reaction by lowering the activation energy without itself being consumed by the reaction
catalyst is regenerated in the process
catalysts provide an alternative reaction pathway with a lower Ea
sometimes the catalysed path contains multiple steps, but each individual step has an Ea that is lower than the overall Ea of the uncatalyzed reaction
catalysts never affect the ∆G of a reaction
Homogenous catalyst
in the same phase as reactants
speeds up the reaction by forming a reactive intermediate
ex. chlorine radicals catalyse the decomp. of ozone
. Cl(g) + O3(g) → ClO(g) + O2(g)
. ClO(g) + O(g) → Cl(g) + O2(g)
Heterogenous catalyst
in a different phase from that of the reactants
most commonly cat= solid, react= gas, liquid
ex. catalytic converter in cars (Pt, and Rh)
2CO(g) + O2(g) —Pt—> CO2(g)
NO(g) —Rh—> N2(g) + O2(g)
Haber-Bosch process
used in the production of NH3 , industries use high pressure and temperature as a catalyst
Enzyme catalysts
enzymes catalyse thermodynamically favourable reactions, causing them to proceed at extraordinarily fast rates
affect reaction rates, but do not affect equilibrium btwn. substrate and products
selectively recognise their substrates over other molecules, resulting in high yields of their products