4 - Electronic Structure of the Atom
1/59
Earn XP
Description and Tags
To be accompanied with past exam questions
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Explain what is meant by energy level
A region of definite energy within an atom that electrons can occupy
Explain what is meant by energy sub-level
A group of atomic orbitals within an atom, all of which have the same energy
Distinguish between the ground state and the excited states of the electron in a hydrogen atom
Ground state: lowest energy / n = 1 / stable //
Excited state: greater energy / n > 1 / unstable
Explain what happens when an electron moves from an excited state to its ground state
Energy is released //
as a photon / as light / at a specific frequency
Name the instrument used to examine the line spectrum of an element
Spectrometer
State Heisenberg’s uncertainty principle
It is impossible to determine the exact position and velocity of an electron at the same time
Write the full s, p electron configuration for an atom of neon (Ne) in its ground state, including the number of electrons in each orbital
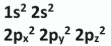
By reference to the electron configuration for neon, distinguish between an energy level and an atomic orbital
Sublevel: 2p // (Ne has) 3
Orbital: e.g. 2px // (Ne has) 5
Neon is chemically unreactive. Explain this property by reference to its electron configuration
Full outer energy level / full p sublevel / satisfies octet rule
Explain how the successive ionisation energy values of neon provide evidence supporting the existence of energy levels
Ninth ionisation energy significantly greater than the eighth
Draw the shape of a p orbital

Define atomic orbital
A region in space where there is a high probability of finding an electron
Explain how the line emission spectrum of hydrogen arises and provides evidence for the existence of energy levels
e- revolve around the nucleus in fixed paths called orbits.
e- s in any orbit have a fixed amount of energy. Energy of an e- in a particular orbit is quantised.
As long as an e- is in any one particular energy level it neither gains nor loses energy.
When energy is supplied to an electron, it is promoted from it’s normal energy level (ground state), to a higher energy level (excited state).
Electrons cannot maintain this excited state and fall back down to their ground state, releasing a definite amount of energy whose value is equal to the difference in energy between the two energy levels.
E2 - E1 = hf {h is Planck’s constant, f is the frequency of light emitted}.
Definite amount of energy appears as a coloured line in the line emission spectrum of the element.
Write the electronic configuration of an atom of sulphur showing the distribution of electrons in atomic orbitals in the ground state.
Hence, state how many
i) main energy levels,
ii) orbitals are occupied by electrons.
1s2 2s2 2p6 3s2 3p4
i) 3
ii) 9
Write the electronic configuration of an atom of calcium showing the distribution of electrons in atomic orbitals in the ground state.
Hence, state how many
i) main energy levels,
ii) orbitals are occupied by electrons.
1s2 2s2 2p6 3s2 3p6 4s2
i) 4
ii) 10
Use the electron configuration of calcium to predict how a calcium atom is likely to behave in a chemical reaction. Explain your reasoning
Loses outer 2 electrons
to satisfy octet rule /
8 electrons in outer shell is stable
The dispositive ion M2+ has 28 electrons and 34 neutrons. What is
i) the atomic number,
ii) the mass number, of M?
i) 30 //
ii) 64
Who coined the name ‘electron‘?
Stoney
What were the contributions of Thomson and Millikan to our understanding of electrons?
Thomson: showed that electrons were negatively charged particles /
identified electrons as subatomic particles //
measured ratio of charge to mass of electron
Millikan: measured size of charge on electron
Give the electron configuration of copper in its ground state
1s2 2s2 2p6 3s2 3p6 4s1 3d10
How are the electrons arranged in the orbitals of the highest occupied sub-level of a nitrogen atom in its ground state?
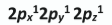
Why does an individual atom not have a definite boundary?
Heisenberg’s uncertainty principle / electron’s position and velocity not known at the same time / wave nature of electron
How is atomic radius defined?
Half the distance between the nuclei of two atoms of an element that are joined together by a single covalent bond
The bond length of the single Cl-Cl covalent bond is 0.199 nm. Predict the value in nm for the atomic radius of chlorine.
0.0995
Account for the general decrease in atomic radii of the elements across the third period of the periodic table from sodium to chlorine
Nuclear charge increasing / atomic number increases / number of protons increases
State and explain the trend in atomic radii across the second period of the periodic table of elements
State: Decrease in atomic radius
Explain: Increase in nuclear charge
Why is establishing an atomic radius for argon problematic?
Ar-Ar bonds are unlikely to exist / do not exist
Define first ionisation energy
The energy required to removed the most loosely bound electron from an isolated atom of an element in it’s ground state
Why do first ionisation energy values show a general increase across the second period of the periodic table?
Increasing nuclear charge //
atomic radius decreasing
Why is the first ionisation energy of potassium lower than that of argon or chlorine?
Outermost electron of potassium is farther from the nucleus than argon or chlorine //
greater degree of shielding (screening) in potassium
Why is the second ionisation energy value for each element in the table bigger than its first ionisation energy value?
Requires more energy to remove electron from a positive ion than from a neutral atom /
greater attraction between the nucleus and electron /
effective nuclear charge increased
Why is the second ionisation energy of potassium very significantly bigger than its first?
Second electron comes from a new shell / first electron removed easily as it is the only electron in outer shell / second electron closer to the nucleus / second electron less shielded
Account for the decreasing trend in first ionisation energy values down Group 1 of the periodic table
Increasing atomic radius / outer electrons farther from nucleus /
extra shell //
additional screening
Why is there a general increase in first ionisation energy values across the elements of the third period?
Nuclear charge increasing //
atomic radius decreasing
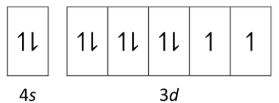
The diagram represents the 10 electrons in the 4s and 3d sub-levels of a neutral atom of a certain element in its ground state.
What is the element?
Nickel (Ni)
Give two differences between an atomic orbit, as described by Bohr, and an atomic orbital
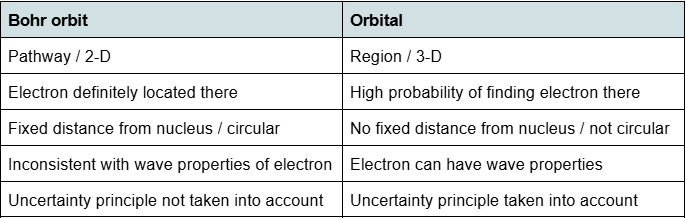
Distinguish between a 2p orbital and a 2p sublevel
2p sublevel consists of three 2p orbitals /
2p sublevel has 6 electrons but each of the 2p orbitals has 2 of these electrons
Use diagrams to distinguish between a p orbital and a p sub-level
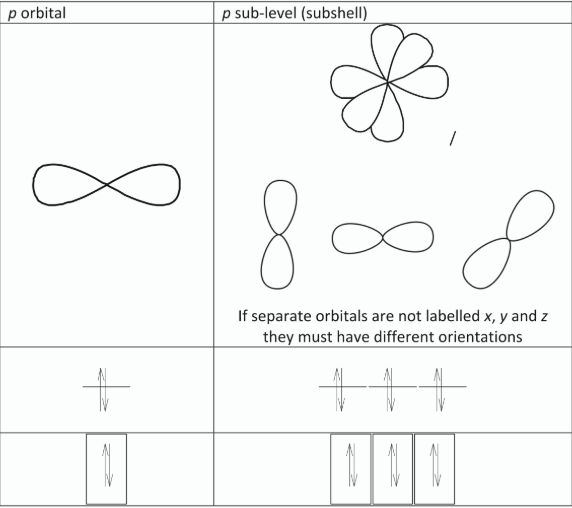
State the maximum number of electrons that can be accommodated in a p-orbital.
2
State one piece of evidence that supports the existence of atomic energy levels
Atomic (emission, absorption, line) spectrum / ionisation energies
Identify the main energy levels involved in the electron transition that gives rise to the first (red) line of the Balmer series in the emission spectrum of the hydrogen atom
3 and //
2
Write the ground state electron configuration for a carbon atom.
How many orbitals are occupied?
1s2 2s2 2p2
4
Explain in terms of energy sublevels why the arrangement of electrons in the main energy levels in a calcium atom is 2, 8,8, 2 and not 2, 8, 10.
4s sublevel is lower in energy than the 3d / electrons fill the 4s sublevel before the 3d
Explain why the first ionisation energy of oxygen is lower than that of nitrogen despite the general increase in values across the second period of the periodic table
Nitrogen is relatively stable //
has a half-filled 2p outer sublevel
or
oxygen is less stable //
doesn’t have half filled 2p outer sublevel
{Note: the next stable state to a full energy sublevel is a half-filled energy sublevel}
The colours produced by fireworks provide evidence for energy levels in atoms of other elements.
Suggest an element that gives a blue-green colour to a firework display
Copper
[Barium acceptable]
Write the electron configuration of an atom of silicon showing the distribution of electrons in atomic orbitals in the ground state.
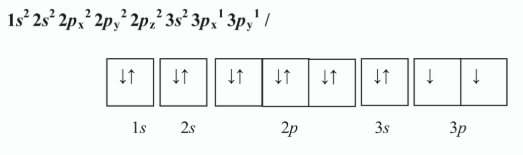
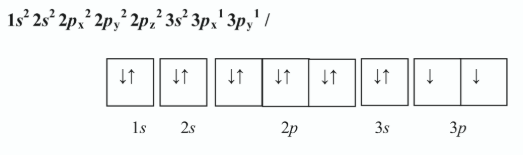
Hence, state how many (i) main energy levels, (ii) atomic orbitals, are occupied in the silicon atom in its ground state
i) 3 //
ii) 8
How many electrons are there in 2.3g of sodium metal, Na?

State Aufbau’s Principle
Electrons will occupy the lowest energy level available to them
State Pauli’s Exclusion Principle
No more than 2 electrons can occupy an orbital and they must have opposite spin
Why is magnesium more reactive than beryllium?
Mg has greater atomic radius / more energy levels in Mg /
more shielding (screening) effect in Mg
Mg has smaller ionisation energy /
What colour is observed in a flame test on a salt of potassium?
Lilac
What colour is observed in a flame test on a salt of sodium?
Orange
What colour is observed in a flame test on a salt of lithium?
Crimson red
What colour is observed in a flame test on a salt of barium?
Yellow / green
What colour is observed in a flame test on a salt of strontium?
Red
What colour is observed in a flame test on a salt of copper?
Blue green
Define electronegativity
The relative attraction of an atom for a shared pair of electrons in a covalent bond
Why is there an increase in electronegativity value moving from gallium to germanium in the periodic table?
Nuclear charge increasing //
atomic radius decreasing
Give one reason why electronegativity values exhibit a general increase across the second period of the periodic table
Increase in nuclear charge / decrease in atomic radius