Cog Neuro - Synaptic Transmission: Chapter 2 Textbook (2.2, 2.6)
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
synaptic transmission
the transfer of a signal from the axon terminal of one neuron to the next
explain chemical transmission
arrival of the action potential at the axon terminal leads to depolarization of the terminal membrane, causing voltage-gated Ca2+ channels to open. This triggers small vesicles to fuse with membrane and release transmitters into the synaptic cleft. different neurons release diff neurons and bind to receptors
what causes Ca2+ to flow into the cell?
action potential depolarization
what does Ca2+ do?
causes vesicles to bind with cell membrane
how are neurotransmitters released into the synaptic cleft?
exocytosis
what are the two types of postsynaptic receptors? explain them
ligand-gated ion channels: where the neurotransmitter (the ligand), in binding to the receptor, leads to opening (gating) of the associated ion cannel. (ionotropic receptors)
g-protein-coupled receptors (GPCRs): where biochemical signals indirectly cause the gating of ion channels that are separate from the receptors themselves: G proteins are those that bind to guanine nucleotides GDP and GTP and act as molecular switches in cells. (metabotropic receptors)
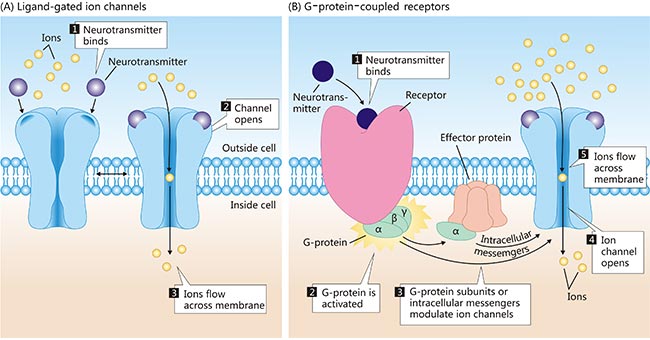
which post-synaptic receptor changes shape?
ligand-gated ion channels
does hyperpolarization of the postsynaptic neuron produce an inhibitory postsynaptic potential or an excitatory postsynaptic potential?
inhibitory
what is depolarization?
when the inside of the cell is excitatory or less negative compared to the outside of the cell
what is hyperpolarization?
when the cell is inhibitory or more negative than the outside of the cell
what is repolarization?
when the cell is becoming less polarized (+ is leaving the cell)
second messenger
a diffusible molecule that triggers intracellular reactions that might lead to modulations of membrane permeability or to gene expression. produced by an enzyme. involved in GPCR mediated signaling
which takes longer ligand or GPCR?
GPCR
what makes a molecule a neurotransmitter?
It is synthesized by and localized within the presynaptic neuron, and stored in the presynaptic terminal before release.
It is released by the presynaptic neuron when action potentials depolarize the terminal (mediated primarily by Ca2+).
The postsynaptic neuron contains receptors specific for it.
When artificially applied to a postsynaptic cell, it elicits the same response that stimulating the presynaptic neuron would.
biochemical classifications of neurotransmitters
amino acids (GABA, glutamate, glycine)
biogenic amine (dopamine, norepinephrine, epinephrine, serotonin, and histamine)
acetylcholine
neuropeptides
what are the five groups of neuropeptides
tachykinins
neurohypophysial hormones
hypothalamic releasing hormones
opioid peptides
other peptides
do all neurons produce one type of neurotransmitter
some produce multiple while others produce multiple
what biogenic amine neurotransmitter systems of the brain produces dopamine?
substantia nigra and ventral tegmental area
what biogenic amine neurotransmitter systems of the brain produces norepinephrine?
locus coeruleus
what happens when a neurotransmitter system is activated?
large areas of the brain can be affected because the transmitter system has diffuse neuronal projections in the brain
what biogenic amine neurotransmitter systems of the brain produces histamine?
tuberomammillary nucleus of hypothalamus
what biogenic amine neurotransmitter systems of the brain produces serotonin?
raphe nucleus
will the same neurotransmitter have the same effect on different post-synaptic cells?
no, the same neurotransmitter on a different neuron may inhibit or excite the neuron. depends on the receptor.
what are some excitatory neurotransmitters?
ACh, catecholamines, glutamate, histamine, serotonin, neuropeptides
what are some inhibitory neurotransmitters
GABA, glycine, some of the neuropeptides
what are the primary players in balancing between excitation and inhibition?
glutamate (most prevalent, excitatory) and GABA (second most prevalent, inhibitory)
what releases glutamate?
pyramidal cells of the cortex, most common cortical neurons
what is linked to too much glutamate in the brain?
stroke, epilepsy, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s
what does the most common GABA receptor do?
open Cl- channels to allow an influx of negatively charge ions into the cell hyperpolarizing the membrane, inhibiting the neuron by making it much less likely to fire.
what is linked to too much GABA in the brain?
seizures, increased emotional reactivity, heart rate, bp, food + water intake, sweating, insulin secretion, coma ect.
what does ACh do?
acts as a neurotransmitter and neuromodulator and supports cognitive function
what does ACh bind to and where are these receptors located?
nicotinic (in autonomic ganglia) and muscarinic (in the CNS, heart, lungs, GI tract and sweat glands
what does excess ACh do?
bad: rigor mortis, death (muscle receptor)
good: increase arousal, sustain attention, enhance learning and memory, and increase REM sleep.
what are some functions of dopamine?
cognitive and motor control, motivation, arousal, reinforcement, and reward.
what are some come consequences of deficits in the dopamine system?
Parkinson’s disease, schizophrenia, ADHD, additction
what kind of receptor does serotonin use? (ligand or GPCR)
both
what is serotonin for?
regulation of mood, temperature, appetite, behaviour, muscle contraction, sleep, and the cardiovascular and endocrine system also learning and memory.
where do SSRIs work?
raphne nuclei
where is locus coeruleus located?
the pons
what kind of receptors exist for norepinephrine?
GPCRs (both inhibitory and excitatory)
pros and cons of norepinephrine
pros: arousal, alertness, vigilance, focus, enhanced memory formation, increases ability to store glucose for energy
cons: increased anxiety, restlessness (increased hr, bp, bf to skeletal muscles, and decreased bf to GI systems)
neurosteroids
involved in the control of cognition, stress, anxiety, depression, aggressiveness, bt, bp, locomotion, feeding behaviour, sexual behaviour
what is the point of removing neurotransmitters and how is it done?
to prevent further excitatory or inhibitory signal transduction. it is done by active reuptake back into pre-terminal or diffusion
what are autoreceptors?
located on presynaptic terminal and bind with the release of neurotransmitters, enabling the presynaptic neuron to regulate to synthesis and release of the transmitter
what does isopotential mean in electrical transmission
the pre and post synaptic cells have the same electrical potential
what are electrical synapses good for?
rapid conduction, activate muscles quickly to get out of harms way.
why is it not advantageous for all of our neurons to be connected as golgi suggested?
because it would take too long to create responses and require way too much energy. very inefficient
what are the two solutions to brain evolution?
minimizing connection lengths: creating short connections to keep processing localized. smaller the neuron, the faster the signal. forms clusters of independent processing units called modules.
retaining a small number of very long connections between distant sites: high degree of local efficiency, and quick communication to the global network. (small-world architecture)
what are the bundle of nerve fibers that connect Broca’s and Wernicke’s area called?
arcuate fasciculus
why might your legs not have fast connection to Broca’s or Wernike’s areas, but have fast connection to the motor cortex?
because when you are in fight or flight mode, you do not need to use your speech and language as much as your motor cortex.
what techniques are used to map and catalog brain areas now?
diffusion-based magnetic resonance imaging, combined with computer algorithms to trace white matter tracts
what are some prominent connection in the brain?
right hemisphere spatial attention network→ the posterior parietal cortex, the frontal eye fields, and the cingulate gyrus
face/object network → lateral temporal and temporopolar connections
cognitive control network → lateral prefrontal, orbitofrontal and posterior parietal cortical connections
default network → posterior cingulate cortex, medial prefrontal cortex, angular gyrus, and their subnetworks