Chem 163 Final
0.0(0)
Card Sorting
1/23
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
1
New cards
Oxidation Reduction Reactions (Redox Reaction)
two 1/2 reactions, transfer of an e-
2
New cards
Oxidation
lose e-
* increases oxidation number
* increases oxidation number
3
New cards
Oxidizing agent
oxidizes another species
* itself is reduced
* itself is reduced
4
New cards
Reduction
gain e-
* reducing oxidation number
* reducing oxidation number
5
New cards
Reducing agent
reduces another species
* itself is oxidized
* itself is oxidized
6
New cards
1. How to assign oxidation number
the number of an atom is 0
* Fe(s) = 0
* Na (s) = 0
* Fe(s) = 0
* Na (s) = 0
7
New cards
2. How to assign oxidation number
the oxidation number of a monatomic ion is its charge
* Na^+ = +1
* Cl^+ = -1
* Al^3+ = +3
* Na^+ = +1
* Cl^+ = -1
* Al^3+ = +3
8
New cards
3. How to assign oxidation number
the sum of the oxidation number in a compound is 0
* Na^+1 Cl^-1 = 0
* Na^+1 Cl^-1 = 0
9
New cards
4. How to assign oxidation number
* Polyatomic ions
the sum of the oxidation numbers is the charge of the ion
10
New cards
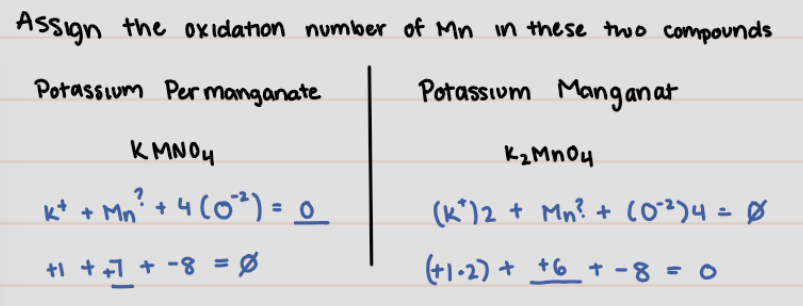
Assign the oxidation number of Mn in these two compounds
* Potassium Permanganate: KMnO4
* Potassium Manganate: K2MnO4
* Potassium Permanganate: KMnO4
* Potassium Manganate: K2MnO4
KMnO4 = +7
K2MnO4= +6
K2MnO4= +6
11
New cards
What is the Oxidation number of Cr in the dichromate ion?
* CrO7 ^2-
* CrO7 ^2-
CrO7 ^2- = -2
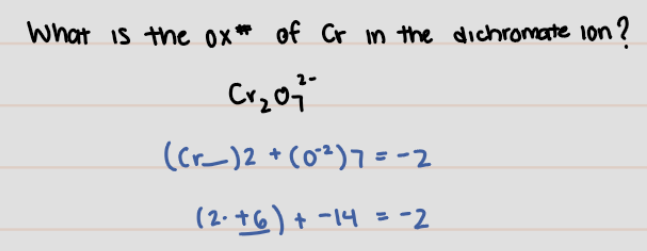
12
New cards
Solid Zinc reacts with aq Silver (I) ion to produce aq Zinc (II) ion and solid silver
Zn(s) + Ag^+ (aq) → Zn^2+ (aq) + Ag(s)
* What is being oxidized?
* What is being reduced?
* Write the 1/2 reactions
Zn(s) + Ag^+ (aq) → Zn^2+ (aq) + Ag(s)
* What is being oxidized?
* What is being reduced?
* Write the 1/2 reactions

13
New cards
Sn^2+ (aq) + Fe^3+ (aq) → Sn^4+ (aq) + Fe^2+ (aq)
* What is the balanced redox reaction
* What is the balanced redox reaction
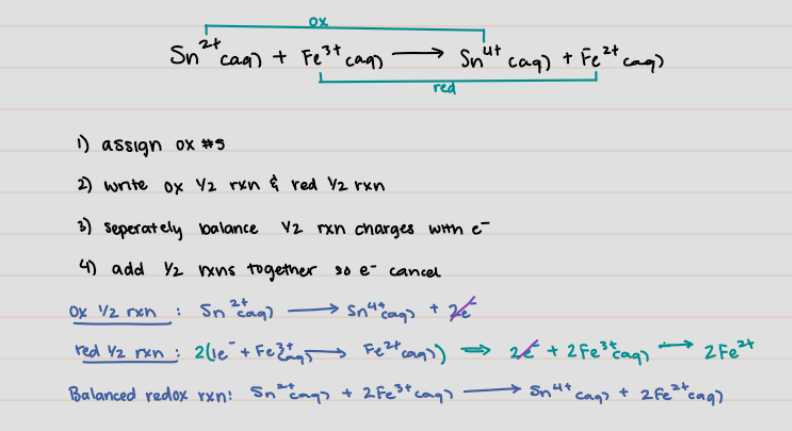
14
New cards
Balancing Redox Reactions in Acidic Solutions Rules
1. Write out the half reactions
2. Balance 1/2 reactions
1. ox 1/2 rxn
2. red 1/2 rxn: # of balance element being reduced
3. Balance oxygen by adding water
4. Balance hydrogen by adding H+ (aq)
5. Balance charge by adding e-
6. Add 1/2 reactions and simplify
\
15
New cards
Fe^-2 (aq) + NO3^- → Fe^3+ (aq) + NO(g)

16
New cards
Cr2O7^2- (aq) + S^2- (aq) → S(s) + Cr^3+ (aq)

17
New cards
Balancing Redox Reactions in Basic Reactions
1. Divide the reaction into half reactions
2. Balance the elements other than H and O
3. Balance the O atoms by adding H2O
4. Balance the H atoms by adding H+
5. Add OH- ions to BOTH SIDES to neutralize any H+
6. Combine H+ and OH- to make H2O
7. Simplify by cancelling out access H2O
8. Balance the charges by adding e=
9. Add the half reactions and simplify
18
New cards
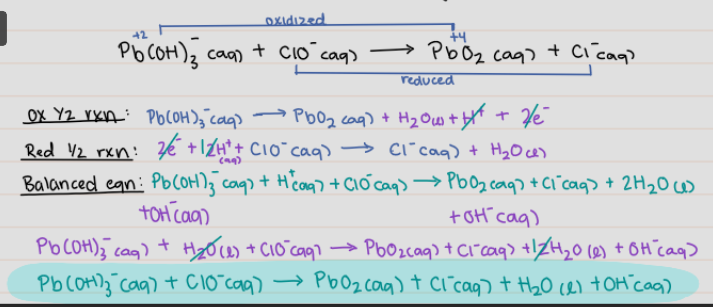
Pb(OH)3^- (aq) + ClO^- (aq) → PbO2 (aq) + Cl^- (aq)
19
New cards
Pb(s) + NO3^- (aq) → Pb ^2+ (aq) + NO2 (aq)
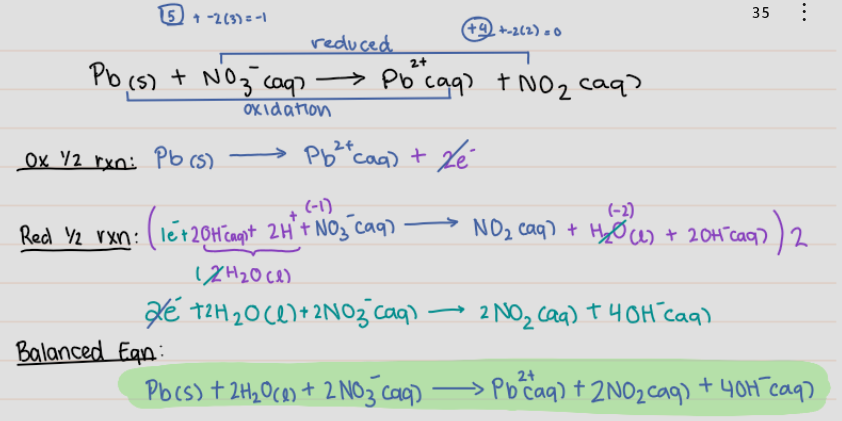
20
New cards
Voltaic or Galvanic Cells
an electrochemical cell in which a spontaneous reaction generates an electric current
* consist of two half cell (1/2 cells)
* electrical connection between the two 1/2 cells
* Salt bridge
* consist of two half cell (1/2 cells)
* electrical connection between the two 1/2 cells
* Salt bridge
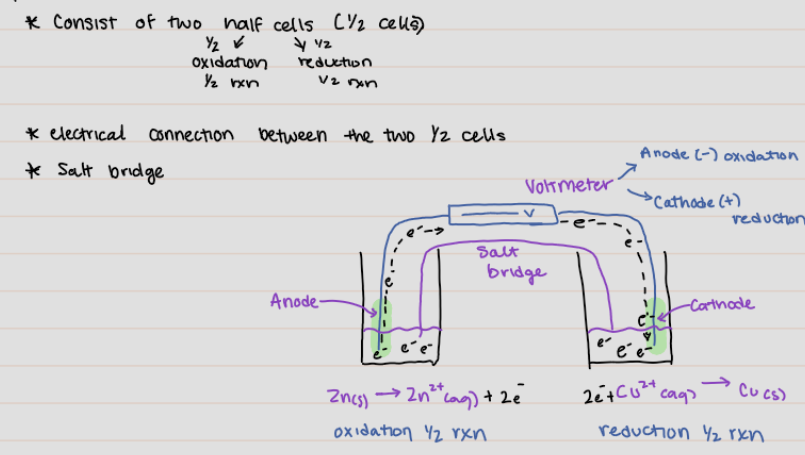
21
New cards
Voltaic Cell Notation
* ox 1/2 left (anode)
* red 1/2 right (cathode)
* phase boundary **I** (single vertical bar)
* salt bridge with two vertical bars II
* red 1/2 right (cathode)
* phase boundary **I** (single vertical bar)
* salt bridge with two vertical bars II
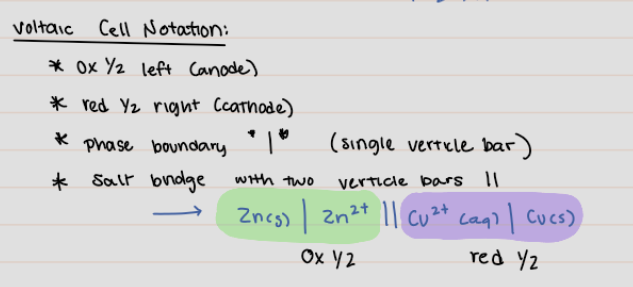
22
New cards
The cell notation for a voltaic cells is as follows:
* Al(s) I Al^3+ (aq) II Cu^2+ I Cu(s)
Write the oxidation and reduction 1/2 reactions and balance then and add them together
* Al(s) I Al^3+ (aq) II Cu^2+ I Cu(s)
Write the oxidation and reduction 1/2 reactions and balance then and add them together

23
New cards
Nernst Equation
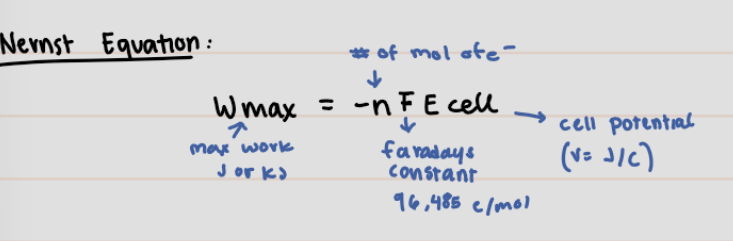
24
New cards
The emf of a particular cell is 0.500 v. the cell reaction is :
* 2Al (s) + 3Cu^2+ (aq) → 2Al ^3+ (aq) + 3Cu (s)
Calculate the maximum electrical work of this cell is obtained from 1.00g aluminum
* 2Al (s) + 3Cu^2+ (aq) → 2Al ^3+ (aq) + 3Cu (s)
Calculate the maximum electrical work of this cell is obtained from 1.00g aluminum
