Isomerism
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
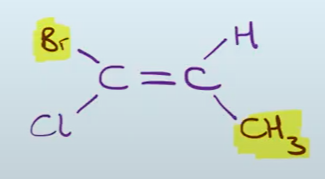
Draw the structure of (E)-1-bromo-1-chloropropene
what two features must a molecule have to show E/Z isomerism?
restricted rotation around the carbon double bond
2 different functional groups attached to each carbon in the carbon double bond
how do you identify the higher priority groups in an E/Z isomer?
priority is based on the higher atomic number
define optical isomers
non-superimposable mirror images
what is another word for optical isomer?
enantiomer
What are the features of a molecule with optical isomers
have a chiral carbon center with 4 different functional groups attached
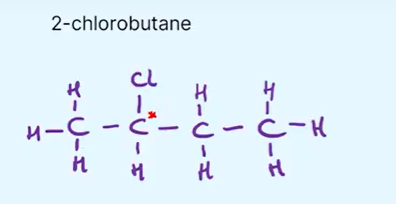
How do you show which carbon is the chiral center in a structural formula?
add an Asterix *
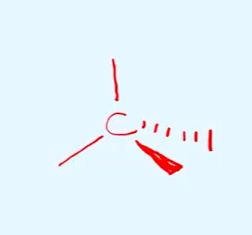
draw the basic structure of all optical isomers, and state the shape and bond angles
tetra hedral
bond angles of 109.5
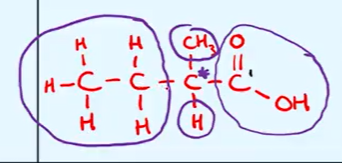
draw out the displayed formula of 2-methylbutanoic acid and identify the chiral carbon
Pentan-2-one and pentan-3-one can both be converted into secondary alcohols, the alcohols produced do not rotate plane polarised light explain this observation (4 marks)
pentan-3-one produces a racemic mixture. As the molecule is planar around the carbonyl group. There is an equal chance for nucleophilic attack above or below the plane.
the products of pentan-2-one do not produce chiral carbons, no optical isomers produced.