Antimicrobials
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
Bactericidal
drug that kills bacteria independent of host immune function
Bacteriostatic
drug that inhibits bacterial growth without killing them. immune system can then more rapidly eradicate the non-multiplying bacteria
Time dependent AMDs
antimicrobials whose effectiveness depends on the duration of exposure to the drug rather than the concentration.
efficacy is associated with length of time drug conc remains above Minimum Inhibitory Concentration (MIC)
levels should remain above MIC throughout course of therapy
Time dependent AMD groups
beta-lactams, tetracyclines,TMS, macrolides
Concentration dependent AMDs
are antimicrobials whose effectiveness is primarily related to the peak concentration achieved in the body. Higher concentrations lead to a more effective bacterial kill.
efficacy depends on peak conc
not necessary to maintain levels above MIC for the entire interval between doses
Conc dependent examples
Aminoglycosides, fluoroquinolones, metronidazole, azithromycin
Why do we not give antimicrobials to ruminants?
Antimicrobials can disrupt the normal gut flora of ruminants, leading to digestive issues and resistance development. Affect the fermentation process essential for ruminant digestion.
Why do we not give antimicrobials to hind-gut fermenters?
toxins are lethal to the beneficial gut microbiota, disrupting digestion and nutrient absorption
What bacteria are sensitive to Penicillin G?
Gram + aerobes, Anaerobes
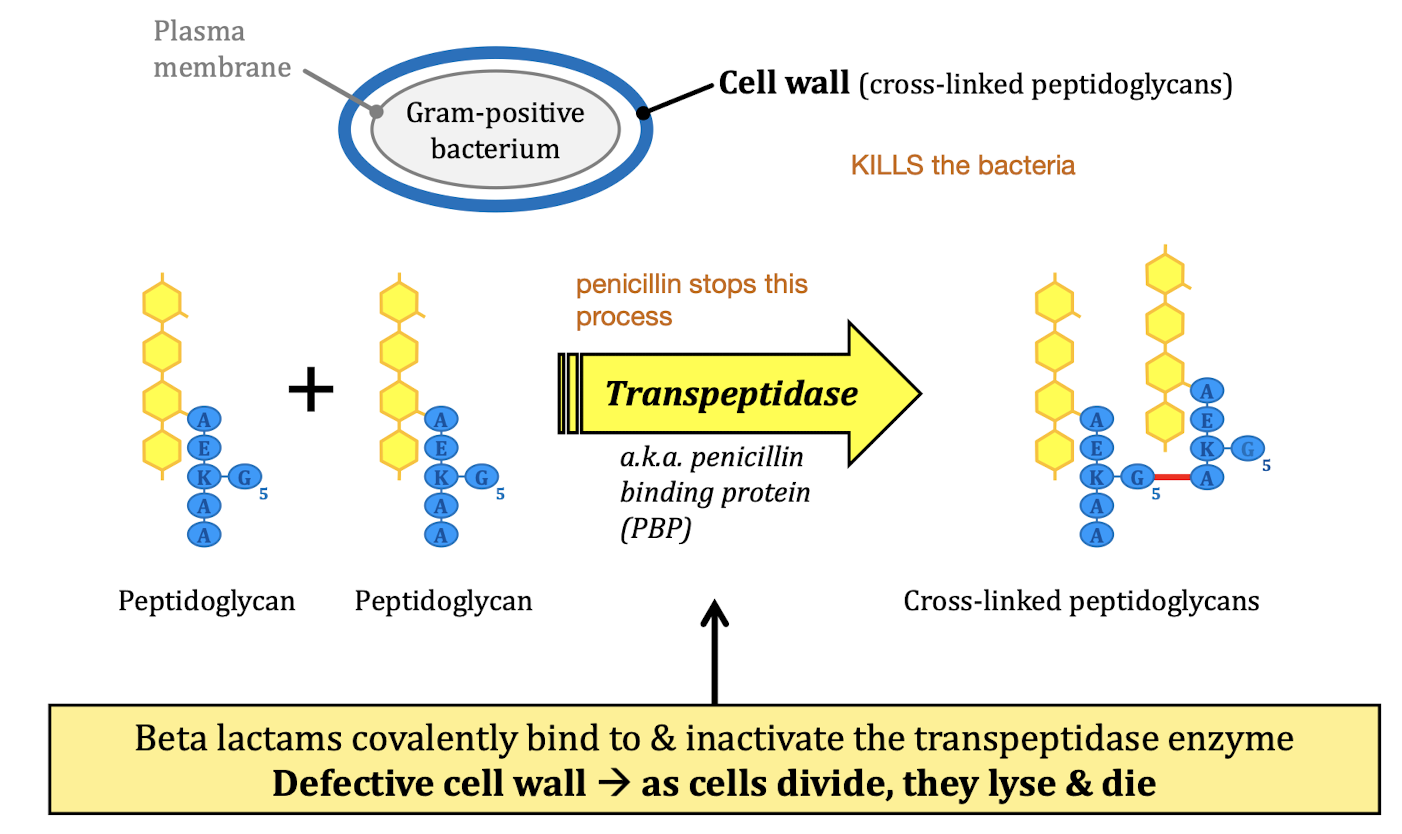
MOA: Inhibit cell wall synthesis
Beta-lactams
MOA: Inhibition of protein synthesis
Aminoglycosides, tetracyclines, macrolides
MOA: Damage to DNA
Fluoroquinolones
MOA: Inhibition of folic acid synthesis
Sulfonamides
MOA: Damage to plasma membrane
Many antifungals
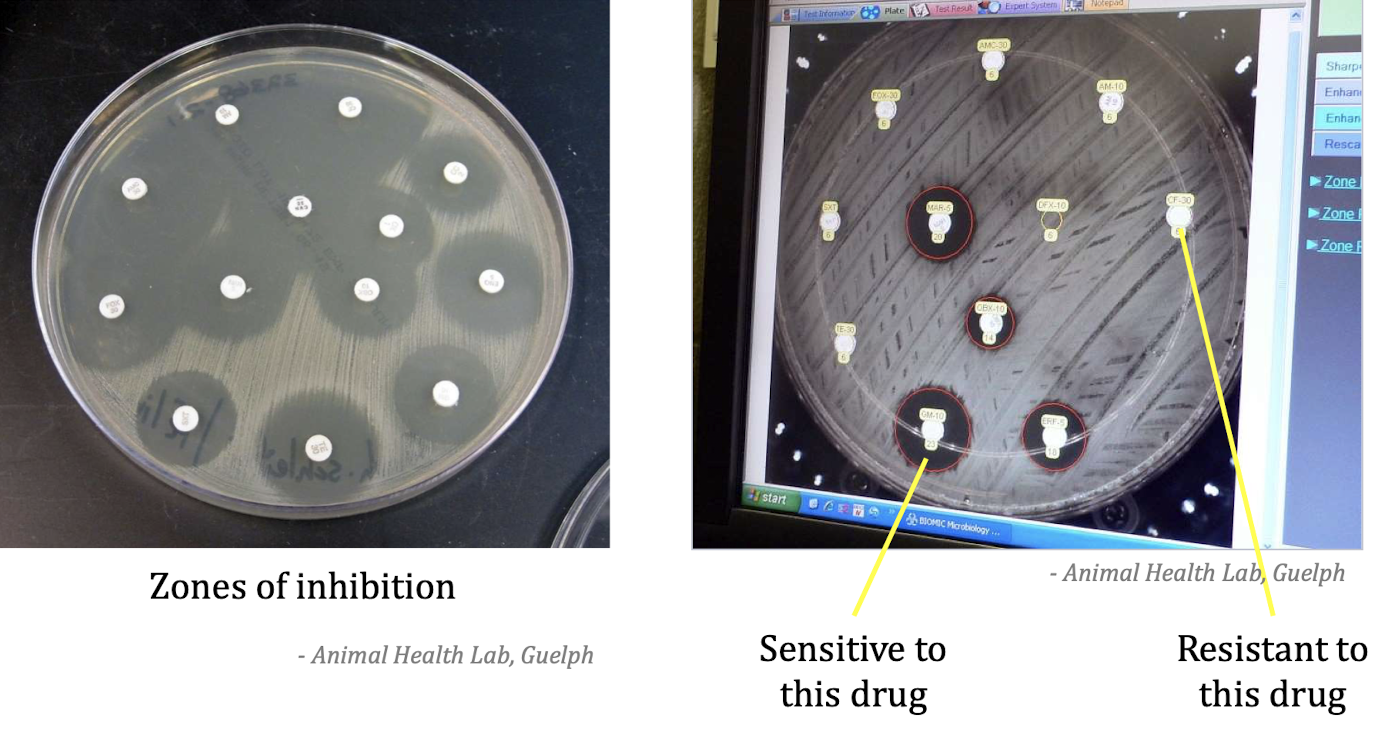
How do we perform a Kirby-Bauer test?
In the Kirby-Bauer test, inoculate a Mueller-Hinton agar plate with the bacterial isolate, place antibiotic disks on the surface, incubate at 35-37°C for 18-24 hours, and measure zones of inhibition.
Results of the Kirby-Bauer test
Results indicate bacterial sensitivity or resistance to antibiotics based on the size of the inhibition zones: larger zones suggest sensitivity, while smaller zones indicate resistance.
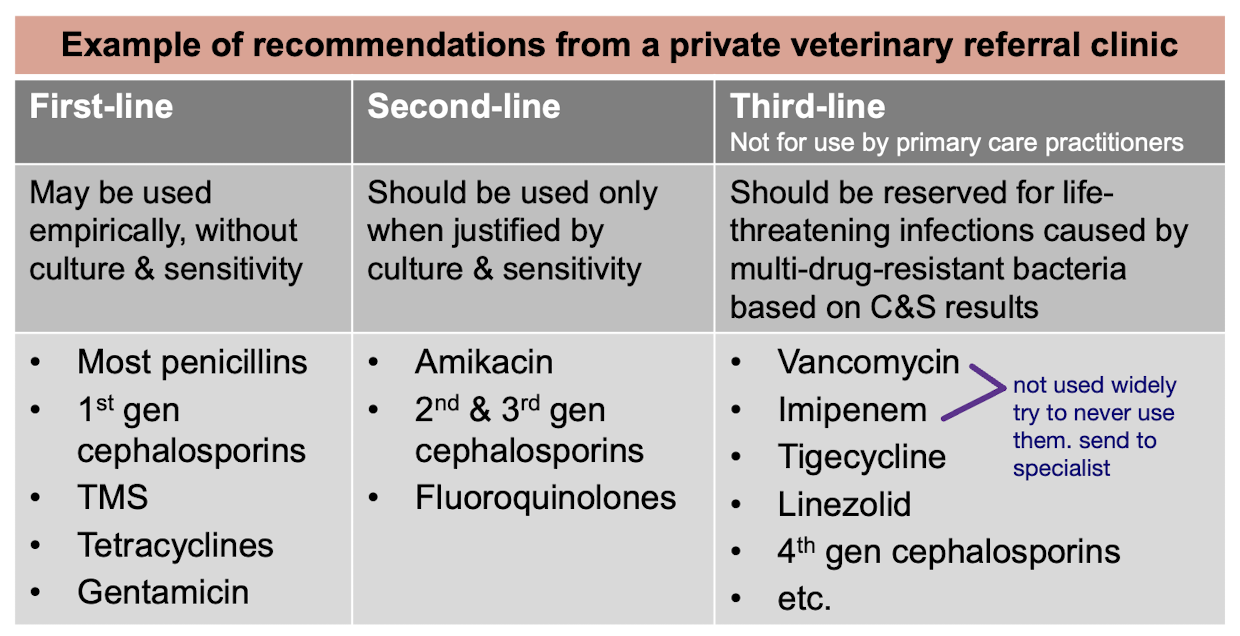
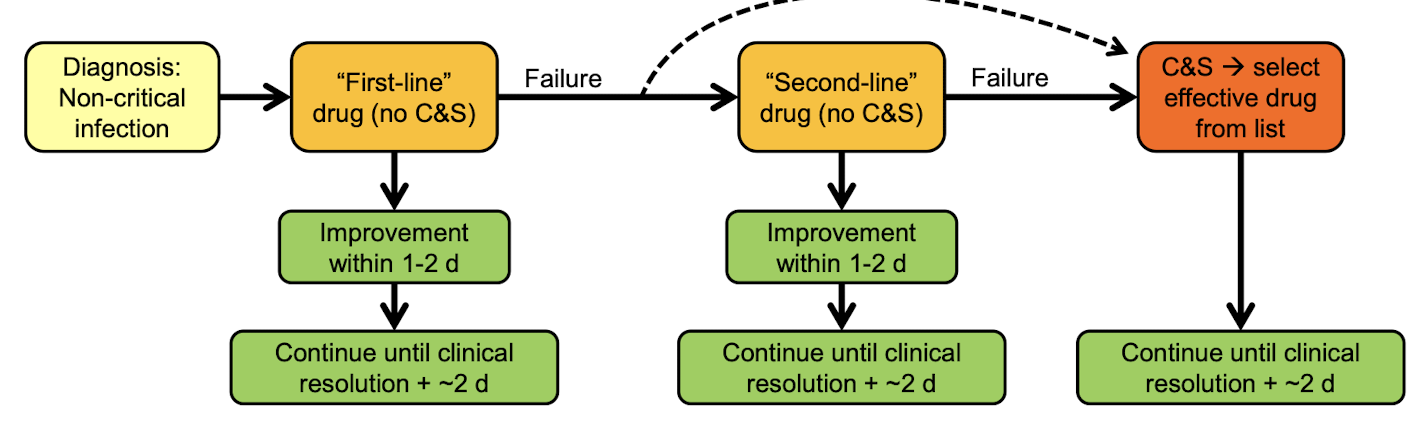
What to do if your antibiotic treatment is not working?
cytology & knowledge of the most common pathogens
Culture & Sensitivity testing
Life threatening infections
Non-critical resistant infections
When selecting a drug what should we consider?
Bacterial sensitivity → want narrow spectrum and ones that avoid for commensal bacteria
Bacteriostatic vs. bactericidal
Adverse effects → outweigh likely adverse effects
Distribution → make sure drug gets to site of infection
Cost
When should we use antibiotic when there is no infection?
Osteomyelitis → avascular bone fragments
Foreign body
Implants
If an abscess is localized with no pyrexia or neutrophilia, do we need to give systemic antibiotics?
May not be needed - give if suspicious that bacteria have traveled
What are some intracellular pathogens?
Mycobacterial, salmonella, legionella
requires drug that enters the cells
When could we need drug dose higher than normal or for longer?
Dysfunction host defense
When do we use prophylactic antibacterials?
high risk following trauma
patients conditions
surgery → opening GI, head & neck, joint, c-section, thoracic
Beta-Lactam class
Penicillin - related to Cephalosporins
ring kills the bacteria
-cillin
effective growing bacteria
warming or freezing can destroy BL ring
very safe
resistant bacteria have: penicillinase or beta-lactamases
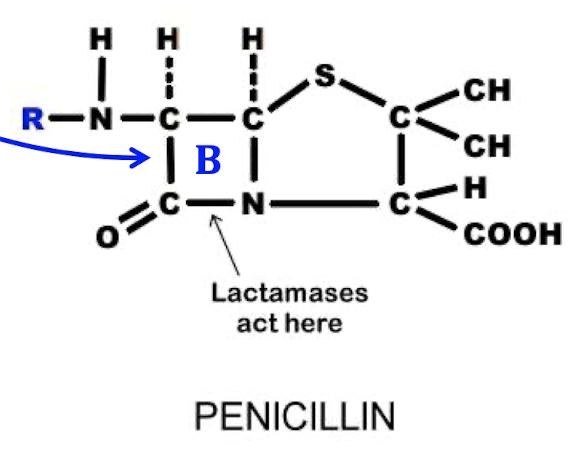
What bacteria are killed by Penicillins?
Gram + → due to unprotected peptidoglycan layer
Pharmacokinetics of Penicillins
distribute well to extracellular fluids everywhere (not CNS & prostate)
Acids stability varies
Short half-life
Elimination: no metabolism → excreted intact in urine → renal excretion of unaltered drug → good for bladder infections
What are the most common adverse effect of Penicillins
Hypersensitivity → mild to anaphylactic, if allergic to 1 then allergic to them all
Colitis in hindgut fermenters → do not give orally
Breakthrough seizures in epileptics → beta-lactams inhibit GABA in the brain → inc excitability
What bacteria are resistant to Penicillins?
Gram -
→ they have penicillinase, plasma encoded can be transferred to other bacteria
What bacteria are sensitive to Penicillin G?
Gram + aerobes
Anaerobes
Sodium
30 min half life
Procaine
depot formulation → slower release, longer action
Not acid stable
What bacteria are sensitive to Amoxicillin?
Gram + aerobes
Anaerobes
Slight Gram - aerobes (enterobacteriaceae)
oral bioavailability 90%
Penicillinase inhibitor: clavulanic acid + amoxicillin
clavamox
Health Canada prudent use stance for Penicillins
First line: Penicillin G, Amoxicillin
Second line: Potentiated penicillins
How are Cephalosporins different from penicillins?
Most are not acid stable → not effective if administered orally
Gram - aerobes
drugs enter CNS reasonably well
drugs metabolized
more resistant to beta-lactamases
less likely to cause allergic reactions in patients with penicillin allergies
What bacteria will 1st generation Cephalosporin act against?
Gram + aerobes
Anaerobes
Gram - aerobes
First line
What is a common oral cephalosporin?
Cephalexin
What are some common parenteral cephalosporins?
Cefazolin → surgical prophylaxis
Cephapirin → IM cattle
What bacteria will 3rd generation Cephalosporin act against?
Gram - aerobes (enterobacterial)
Gram + aerobes
Anaerobes
Ceftiofur → resp infection, foot rot, metritis, UTI
Second line
Cefovecin (convenia)
SQ inj
skin & resp infections in dogs and cats
very long half-life → 2 weeks formulation
not good for infections that will be resolved in a few days
inappropriate use for short-term
Cefpodoxime proxetil might be better second line drug use
Adverse effects with cephalosporin
hypersensitivity
oral may cause colitis in hindgut fermenters
reduction of seizure threshold
PK features of cephalosporin
few are acid stable
not destroyed by penicillinases but may be beta-lactamases
some 3rd gen drugs enter CNS readily
How can we use Aminoglycosides?
-micin, -mycin
Topical → First line, Staph
Systemic → Second line, for dangerous Gram - aerobic infections, life-threatening conditions
atypical → mycoplasma
Highly ionized → limited ability to cross membranes, negligible oral or topical absorption
What is the most common Aminoglycoside?
Gentamicin → given parenterally
MOA of aminoglycosides
Inhibit bacterial protein synthesis → inhibition is incomplete → cause wrong AA to be place in growing protein → act as lethal porins → kills bacterial cell
What bacteria do Aminoglycosides affect the best?
Gram - aerobe
Gram + aerobes → Staph (MRSA/MRSP)
Atypical bacteria → mycoplasma
Why are aminoglycosides ineffective against anaerobes?
entry of drug requires oxygen → absent in anaerobes → do not use for anaerobe bacteria
What are resistant to aminoglycosides?
Anaerobes
Plasmid ac
What are the main adverse effects of Aminoglycosides?
Nephrotoxicity → do not give to patients that are dehydrated or has renal disease
Neomycin is the worst
AGs accumulate in renal tubular cells → enhance free-radical formation → damage some renal tubular epithelial cells in every patient
Ototoxicity → damage to CNVIII & hair cell in cochlea & vestibular apparatus
can cause hearing loss
before putting it in the ear, ensure the tympanum is intact
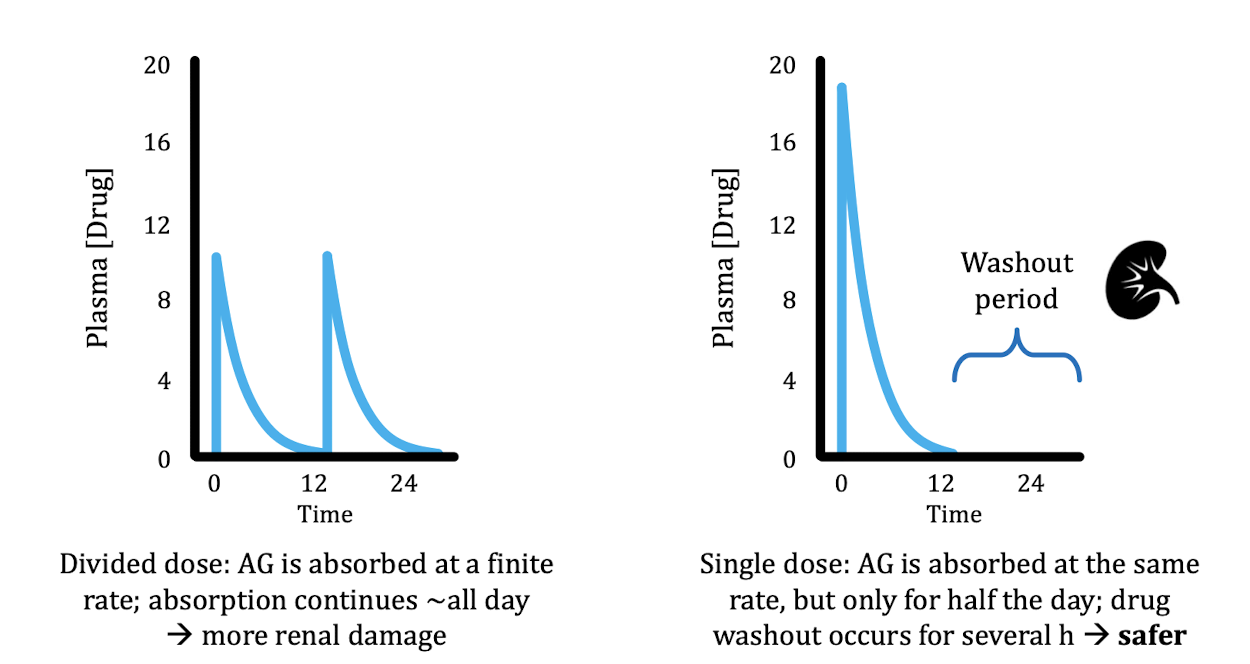
Why should we not give aminoglycosides in two dose instead of one big dose?
Pump does not work very fast → the same amount of drug will be taken up regardless of initial dose → one big dose is safer, allows washout period
Can we give IM aminoglycosides to food animals?
NO → IM use of gentamicin in cattle may result in drug residues detectable for > 1yr
What are second line Aminoglycosides use for infections after gentamicin does not work?
Tobramycin
Amikacin → resistant to many aminoglycosides
What are Tetracyclines used to treat?
atypical bacteria(Rickettsia, Chlamydia, Mycoplasma) in all species → good for livestock
-cycline
MOA of tetracycline
Binds to bacterial ribosomes → inhibit to protein synthesis → bacteriostatic(does not kill, stops bacteria production)
What bacteria do Tetracyclines target?
Small → Atypical bacteria
Large → Atypical bacteria, Gram + & - aerobes, Anaerobes
Absorption of Tetracycline
Divalent cations in food can inhibit oral absorption → cannot give this drug with food
oral bioavailability is low → doxycyline has better bioavailability than most other tetracyclines
Distribution of Tetracycline
Good for extracellular fluid compartment (not CNS) does not work for intracellular bacteria (except doxycycline)
Lipid solubility → doxycycline is most lipid sol → enters host cells well including prostate & CNS
They bind to multivalent cations like calcium, become incorporated into growing bone and teeth
Food animal residue concern
Elimination of Tetracycline
excreted primarily by glomerular filtration in Urine
doxycycline metabolized in liver → not good for UTI → eliminated almost entirely through the intestinal tract into feces
What are the 2 common resistances bacteria have against Tetracycline?
Efflux pump → removes drug from bacterial cell, can get overwhelmed with high drug concentration
Ribosomal protection protein
What are adverse effects of Tetracycline
Incorporation into growing bone & teeth → food animal residue
Nephrotoxicity → do not give to dehydrated animals
Tissue irritation → very painful to administer and may vomit with oral administration
Esophageal lesions from doxycycline in cats → pill is too big → esophageal lesion & strictures in cats
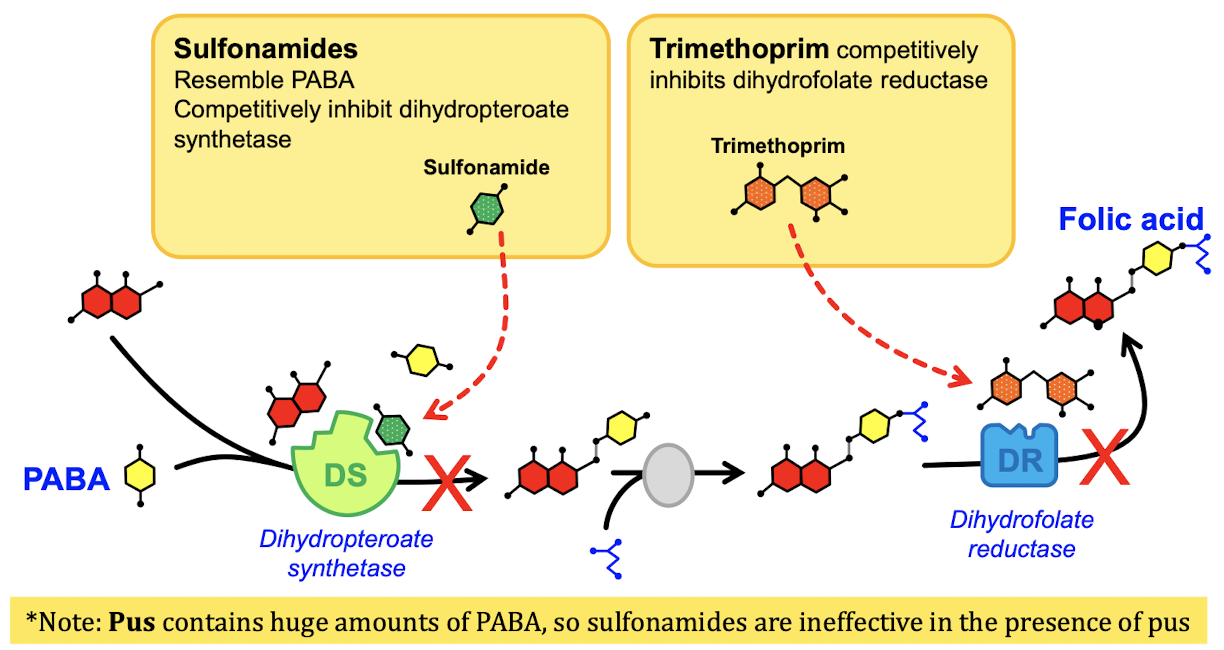
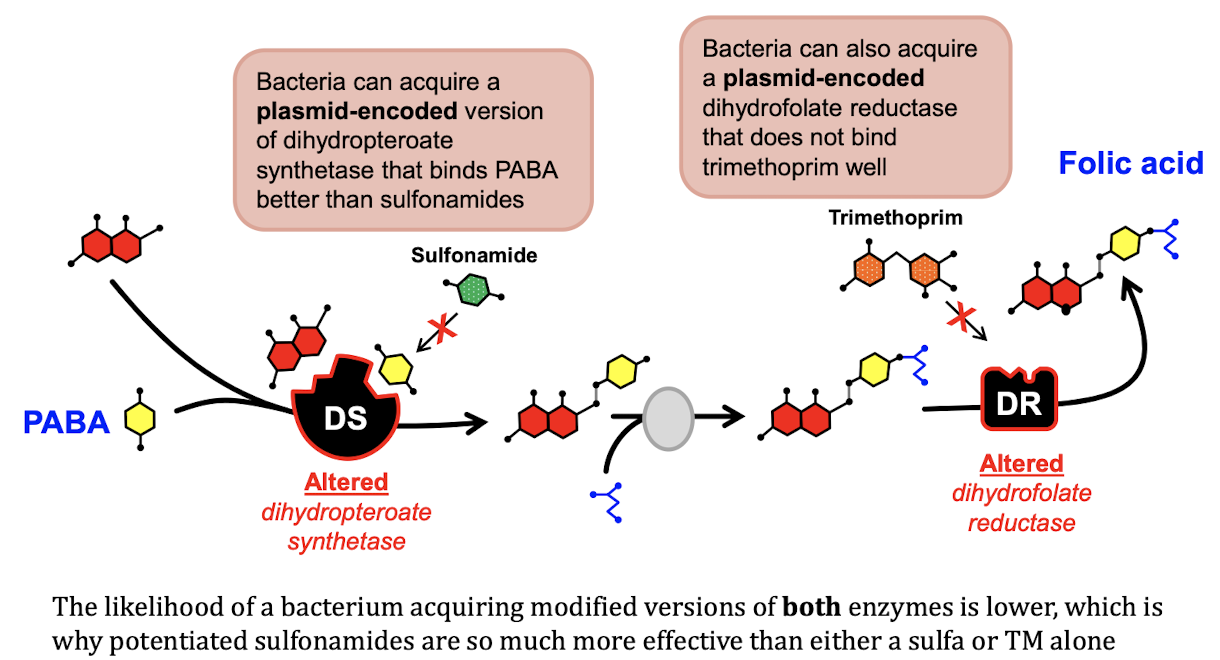
Why do we combine other drugs with Sulfonamides?
Creates a formula that is a bactericide, sulfonamide alone does not kill the bacteria → TMS (trimethoprim sulfonamide)
MOA of Sulfonamides
Inhibit the synthesis of folic acid in the bacteria by binding to dihydropteroate synthetase → do not use these drugs for pus
What bacteria do Sulfonamides target?
Small → Atypical bacteria
Large → Atypical bacteria, Gram + & - aerobes, Anaerobes
Used in humans and small animals for sporadic bacterial cystitis where high concentration of drug in urine overwhelms resistance mechanisms → used for bladder infections
Absorption & distribution for Sulfonamides
good oral absorption
distributes well to all tissues
pus reduces efficacy → don’t use
Elimination of Sulfonamides
renal excretion and hepatic metabolism
renal damage severe in dehydrated patient
Resistance to Sulfonamides
Bacteria can encode plasmid encoded proteins to block drug site
Adverse effects of Sulfonamides
Allergenic than most drugs
Cause dry eye in dogs → They destroy lacrimal tissue in dogs → KCS(keratoconjunctivitis)
Nephrotoxicity in dehydrated patients
What drug classes cause Nephrotoxicity?
DO NOT give to dehydrated animals!!
Sulfonamides, Tetracycline, Aminoglycocides(will always cause renal toxicity, only give to animals that will die without it)
What are the safest class of antibacterials to use?
Macrolides → they sting very badly though
Pharmacokinetics of Macrolides
penetrate cells very well
concentrate in lung & lung macrophages
have long half-lives (erythromycin have short half life)
weak bases
-osin or -mycin
MOA of Macrolides
Inhibit protein synthesis → bacteriostatic effect → bactericidal if conc high enough
What bacteria do Erythromycin target?
Broad spectrum → First line
Gram + aerobes
Anaerobes
Gram - aerobes
Atypical
If you have an animal that has a lung infection and is known to be intracellular, what drug class do we use?
Erythromycin → Macrolides
What enzymes does Erythromycin inhibit?
P450 → other drugs administered might accumulate and cause problems
Stimulate motilin receptors
What bacteria do Macrolides target?
Second line
Gram - aerobes → most effective
Gram + aerobes
Anaerobes
Atypical
Longer half life → bacterial pneumonia
What is Tilmicosin used for?
Macrolides
beef, lambs, rabbits
bacterial pneumonia
Gram - aerobic lung pathogens
DO NOT GIVE IV
do not give sc to swine
What is Tulathromycin/Gamithromycin used for?
Macrolides
bacterial pneumonia
Gram - aerobic
What do bacteria have that cause resistance to Macrolides?
Plasmid encoded efflux pump
Adverse effects of Macrolides
Tissue irritation → IM pain, vomiting w/ erythromycin
Oral toxicity in herbivores → fatal rumen stasis, colitis in hindgut fermenters
Tilmicosin can cause rapid cardiac fatalities when given parenterally
Where do we use Fluoroquinolones?
Second-line use → use when other drugs have failed
Small animals & exotics (some in horses and food animals)
High oral bioavailability, long half life, good tissue penetration
What are some Fluoroquinolones?
-floxacin
Enrofloxacin
Orbifloxacin
Marbofloxacin
Difloxacin
Danofloxacin
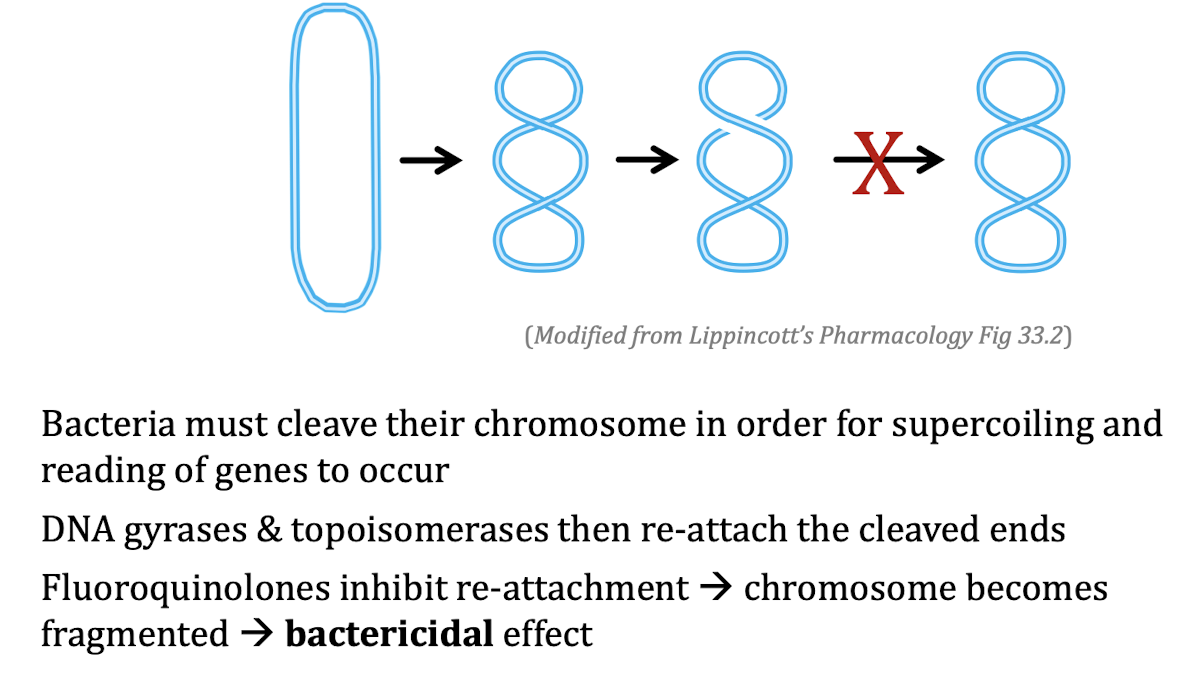
MOA for Fluoroquinolones
Damage DNA → inhibit DNA gyrase & topoisomerases → bactericidal effect
What bacterial do Fluoroquinolones target?
Gram - aerobes
Atypical bacteria
Gram + aerobes → staph
Absorption of Fluoroquinolones
well absorbed orally by monogastrics → close to 100% bioavailability
Distribution of Fluoroquinolones
Goes well to the lungs, and most other tissues including the prostate
Poor CNS, skin distribution
Elimination of Fluoroquinolones
Depends on the drug → long half life
Adverse effects of Fluoroquinolones
Cartilage damage → young animals are highly sensitive → do not give to puppies and foals
Retinal degeneration in cats → high doses
Can trigger seizures in epileptics → FQ inhibit NT GABA
Resistance to Fluoroquinolones
sudden & stable due to mutations in bacterial DNA gyrase/topoisomerase
some bacteria can be resistance to one or all FQ
plasmid born mechanisms
What can Chloramphenicol cause in humans?
Fatal idiosyncratic aplastic anemia in humans
not dose related
genetic predisposition
can develop weeks after therapy
Banned in food animals
What category should we not use in vet med?
Category 1
What drugs does health canada say to use?
Tetracyclines
What antibiotics should we avoid using when possible?
Fluoroquinolones, Potentiated Penicillins, 3rd gen Cephalosporins
What are some examples of AMDs use in general practice?