Inorganic solids equations
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Born Haber Cycle Equation
ΔHf∘=ΔHsublimation+IE+1/2D+EA+ΔHlattice
Where:
ΔHf∘ = Standard enthalpy of formation
ΔHsublimation = Sublimation energy of the metal
IE = Ionization energy of the metal
D = Bond dissociation energy of the nonmetal molecule
EA = Electron affinity of the nonmetal
ΔHlattice = Lattice energy (often determined indirectly)
General steps of the Born Haber Cycle
Sublimation of the metal
Ionisation of the metal atom
Dissociation of the non metal atom
Electron affinity of the non metal atom (anion)
Formation of the ionic compound
Lattice enthalpy equatio
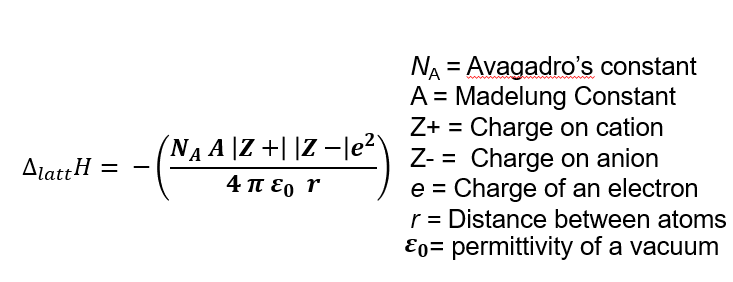
Kapuntskii equation

FCC
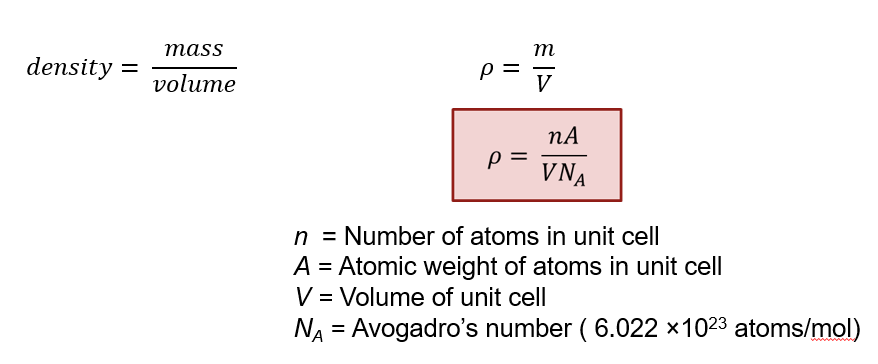
How many atoms in the unit cell?
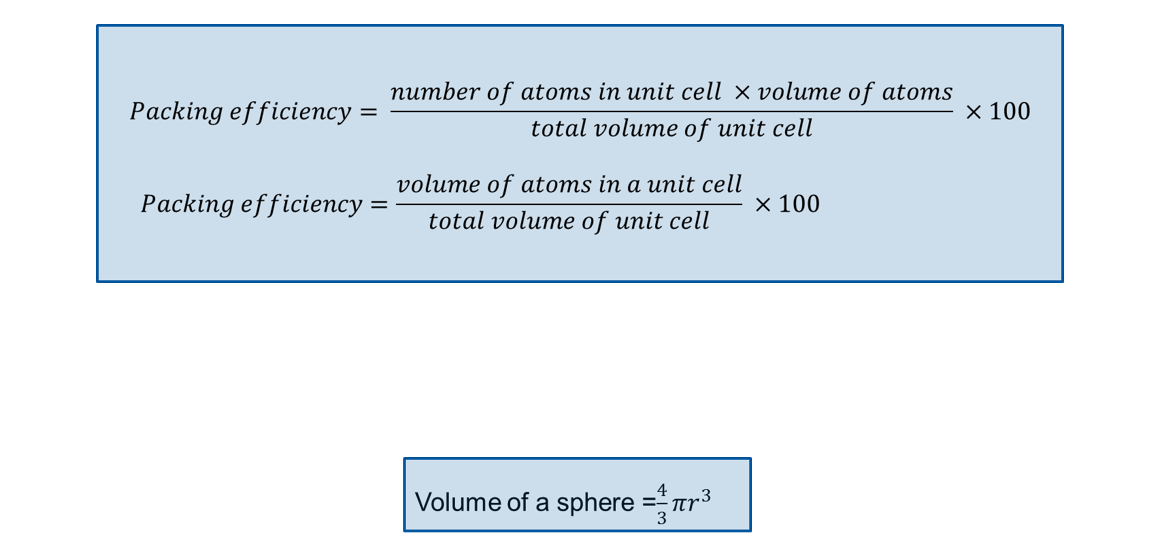
Packing efficiency?
Coordination number?
Where are the atoms placed?
E.g.?
4 atoms
74%
12
Atoms are located at the corners and center of each face of the cube.
Cu, Au, Ag
Simple Cubic Cell
How many atoms in the unit cell?
Packing efficiency?
Coordination number?
Where are the atoms placed?
E.g.?
1 atom
52%
6
Atoms are located at the corners of the cube.
Po, Cs
BCC
How many atoms in the unit cell?
Packing efficiency?
Coordination number?
Where are the atoms placed?
E.g.?
2 atoms
68%
8
Atoms are located at the corners and one atom in the center of the cube.
Fe, Cr
HCP
How many atoms in the unit cell?
Packing efficiency?
Coordination number?
Where are the atoms placed?
E.g.?
6 atoms
74%
12
Atoms are located at the corners of the hexagonal base and in the center of the top and bottom faces.
Mg, Zn
Packing efficiency equation
Unit cell parameter of BCC
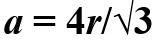
Unit cell parameter of FCC
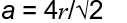
Density equation