MCAT Biochemistry - Carbohydrate Metabolism II: Aerobic Respiration
1/68
Earn XP
Description and Tags
541
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
citric acid cycle / Krebs cycle / tricarboxylic acid (TCA) cycle
oxidation of acetyl-CoA to CO2 and H2O in the mitochondrial matrix; produces the high-energy electron-carrying molecules NADH and FADH2; most substrates and products of the cycle are reused over and over again; will not occur anaerobically
Acetyl-CoA
obtained from the metabolism of carbohydrates, fatty acids, and amino acids; key molecule in the crossroads of many metabolic pathways; high-energy thioester; negative feedback on own production
pyruvate dehydrogenase complex
multienzyme complex located in the mitochondrial matrix
pyruvate dehydrogenase (PDH)
dihydrolipoyl transacetylase
dihydrolipoyl dehydrogenase
^ convert pyruvate to acetyl-CoA
pyruvate dehydrogenase kinase
pyruvate dehydrogenase phosphatase
^ regulate the actions of PDH
inhibited by an accumulation of acetyl-CoA and NADH
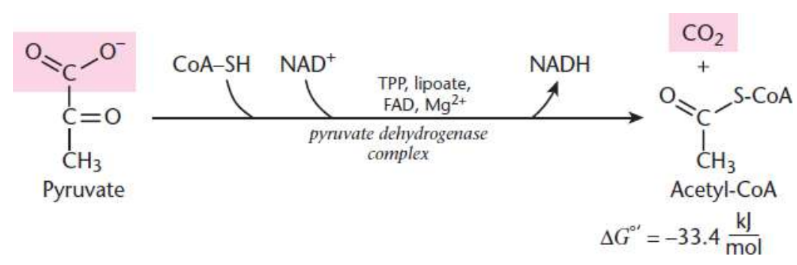
free energy of conversion of pyruvate to acetyl-CoA
exergonic - ΔG∘= −33.4 kJ mol
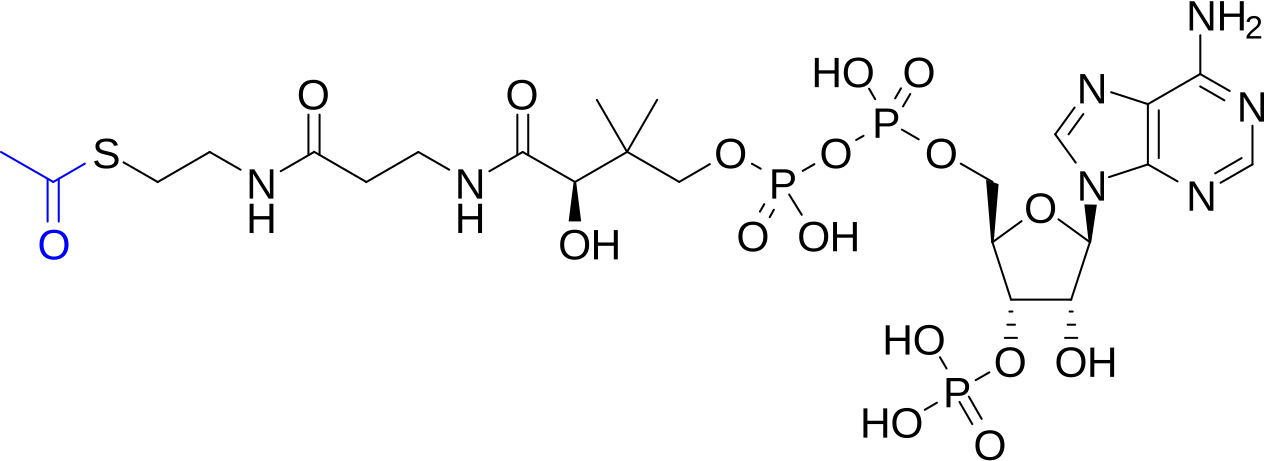
coenzyme A (CoA)
thiol; covalent attachment of the acetyl group to the thiol group → acetyl-CoA = thioester
pyruvate dehydrogenase complex enzymes needed to catalyze acetyl-CoA formation
pyruvate dehydrogenase (PDH)
dihydrolipoyl transacetylase
dihydrolipoyl dehydrogenase
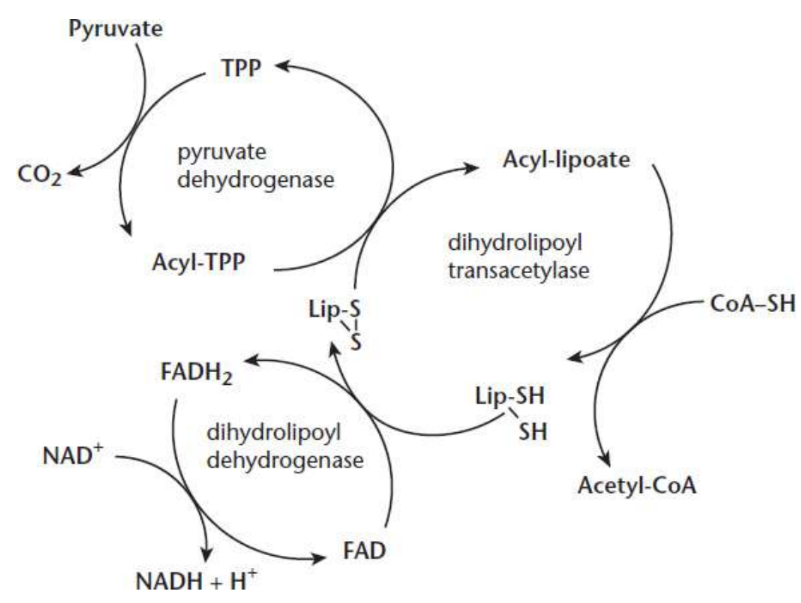
Pyruvate dehydrogenase (PDH)
Pyruvate is oxidized, yielding CO2, while the remaining two-carbon molecule binds covalently to thiamine pyrophosphate; requires Mg2+
thiamine (pyrophosphate, TPP) / vitamin B1
water-soluble vitamin; coenzyme held by noncovalent interactions to PDH
Dihydrolipoyl transacetylase
two-carbon molecule bonded to TPP is oxidized and transferred to lipoic acid, which acts as an oxidizing agent, creating the acetyl group via thioester linkage
catalyzes the CoA−SH interaction with the newly formed thioester link, causing transfer of an acetyl group to form acetyl-CoA; lipoic acid is left reduced
lipoic acid
coenzyme that is covalently bonded to the enzyme
Dihydrolipoyl dehydrogenase
uses FAD to reoxidise lipoic acid; FAD is reduced to FADH2
Flavin adenine dinucleotide (FAD)
coenzyme in order to reoxidize lipoic acid;
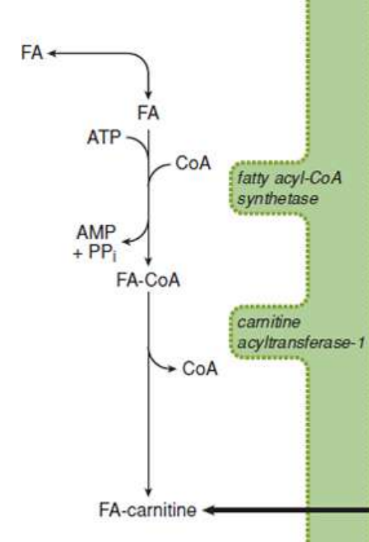
Fatty acid oxidation (β-oxidation)
removes two-carbon fragments from the carboxyl end of acyl-CoA
Carnitine
molecule that can cross the inner membrane with a fatty acyl group in tow; carry the acyl group from a cytosolic CoA to a mitochondrial CoA
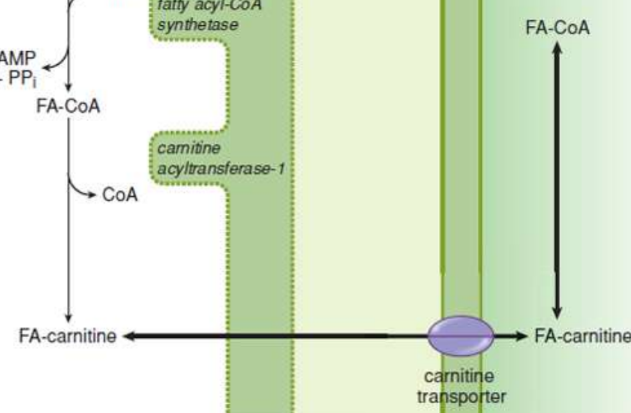
activation (β-oxidation)
in cytosol, causes a thioester bond to form between carboxyl groups of fatty acids and CoA; fatty acyl group is transferred to carnitine via transesterification
Amino acid catabolism
Certain amino acids can be used to form acetyl-CoA via transamination
transamination
transfers an amino group to a ketoacid to form new amino acids; responsible for the deamination of most amino acids
ketogenic amino acids
amino acids that can be metabolised into ketone bodies and subsequently into acetyl-CoA
Ketones catabolism
ketones can turn into acetyl-CoA; reversible
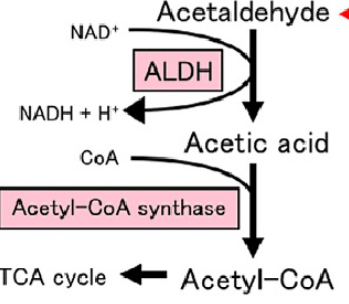
alcohol dehydrogenase
facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH in cytosol; buildup of NADH can inhibit Krebs cycle

excess alcohol metabolism
CYP2E1 catalyzes the buildup of NADH to NAD+
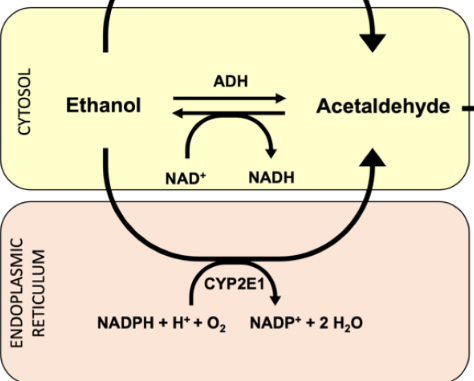
acetaldehyde dehydrogenase
catalyze the conversion of acetaldehyde into acetyl-CoA; often follows alcohol dehydrogenase; used primarily to synthesize fatty acids
Acetaldehyde + NAD+ + Coenzyme A ↔ Acetyl-CoA + NADH + H+
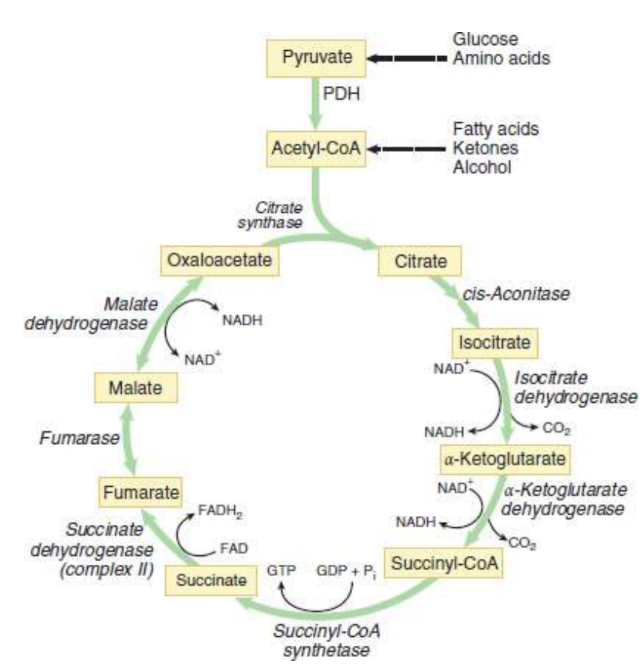
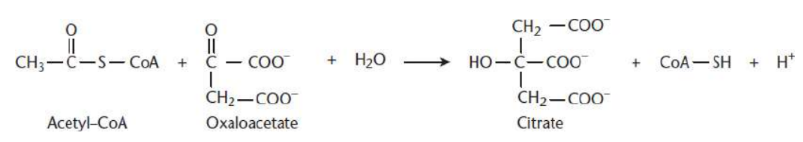
Citrate Formation
first step of Krebs cycle
acetyl-CoA and oxaloacetate undergo a condensation reaction to form citryl-CoA
hydrolysis of citryl-CoA by citrate synthase yields citrate and CoA
energetically favoured step
citrate synthase does not require energy input
Citrate Isomerized to Isocitrate:
second step of Krebs cycle
(achrial) citrate binds at three points to the enzyme aconitase
dehydration yields cis-aconitate
hydration to form (chiral) isocitrate
four possible isomers
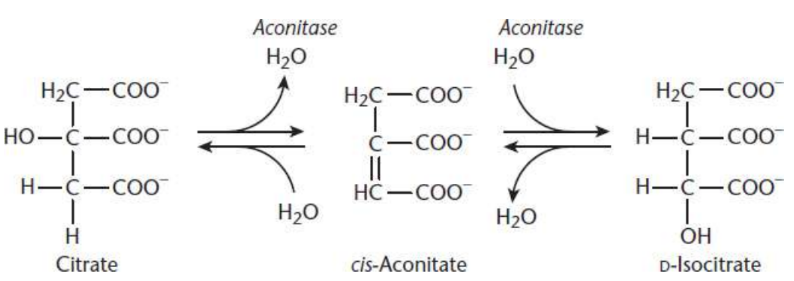
aconitase
metalloprotein that requires Fe2+; Achiral citrate is isomerized to one of four possible isomers of isocitrate via switching of a hydrogen and a hydroxyl group
α-Ketoglutarate and CO2 Formation
third step of Krebs cycle
Isocitrate oxidized to oxalosuccinate by isocitrate dehydrogenase
rate-limiting step
first NADH produced from intermediates in the cycle
oxalosuccinate is decarboxylated to produce α-ketoglutarate and CO2
first of the two carbons from the cycle is lost here
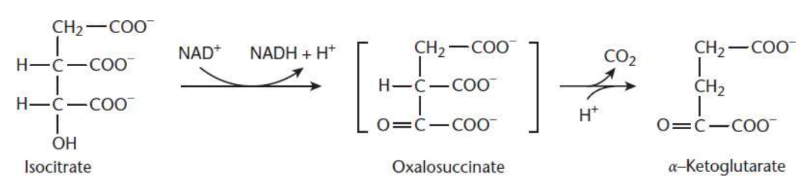
isocitrate dehydrogenase
rate-limiting enzyme of the citric acid cycle; oxidises isocitrate to oxalosuccinate
activated by ADP and NAD+ (allosteric activators)
inhibited by ATP and NADH
Succinyl-CoA and CO2 Formation
fourth step of Krebs cycle
α-ketoglutarate and CoA come together and produce cuccinyl-CoA and carbon dioxide with α-ketoglutarate dehydrogenase complex
second and last carbon lost from the cycle
Reducing NAD+ produces another NADH
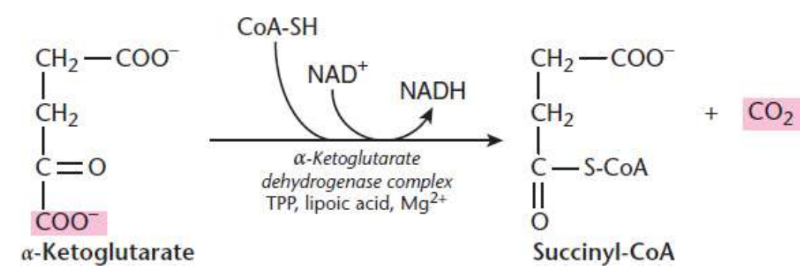
α-ketoglutarate dehydrogenase complex
α-ketoglutarate and CoA come together and produce cuccinyl-CoA and carbon dioxide using thiamine, lipoic acid, Mg2+
stimulated by ADP and calcium ions
inhibited by succinyl-CoA, NADH and ATP
Succinate Formation
fifth step of Krebs cycle
Hydrolysis of the thioester bond on succinyl-CoA yields succinate and CoA by succinyl-CoA synthetase
coupled to the phosphorylation of GDP to GTP
require energy input
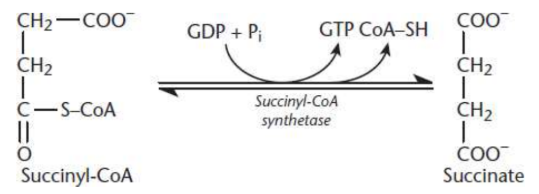
nucleosidediphosphate kinase
catalyzes phosphate transfer from GTP to ADP, thus producing ATP; only time in the entire citric acid cycle that ATP is produced directly
Fumarate Formation
fifth step of Krebs cycle
succinate undergoes oxidation by succinate dehydrogenase to yield fumarate
FAD is reduced to FADH2
occurs in inner mitochondrial membrane
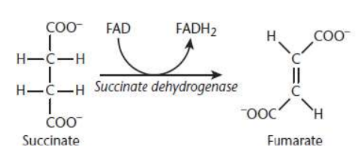
Succinate dehydrogenase
flavoprotein; integral protein on the inner mitochondrial membrane; also part of electron transport
flavoprotein
covalently bonded to FAD, the electron acceptor
Substrates of Citric Acid Cycle Mnemonic
Please, Can I Keep Selling Seashells For Money, Officer?
(Pyruvate
Citrate
Isocitrate
α-Ketoglutarate
Succinyl-CoA
Succinate
Fumarate
Malate
Oxaloacetate)
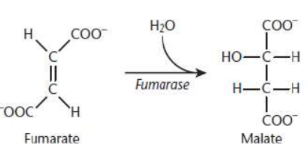
Malate Formation
seventh step of Krebs cycle
fumarase catalyzes the hydrolysis of the alkene bond in fumarate, thereby giving rise to malate
L-malate only
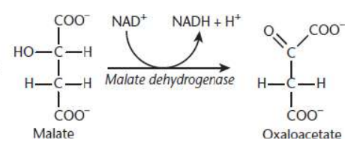
Oxaloacetate Formed Anew
eighth step of Krebs cycle
malate dehydrogenase catalyzes the oxidation of malate to oxaloacetate
third and final molecule of NAD+ is reduced to NADH
Final products of Krebs cycle
4 NADH, 1 FADH2, 1 GTP
NADH ATP conversion
2.5 ATP
FADH2 ATP conversion
1.5 ATP
GTP ATP conversion
1 to 1
pyruvate dehydrogenase kinase
regulation of citric acid cycle via phosphorylation of PDH in response to high ATP; inhibits acetyl-CoA production
pyruvate dehydrogenase phosphatase
PDH dephosphorylated and reactivated in response to high ADP
Citrate synthase
inhibited by citrate, succinyl-CoA, NADH and ATP
electron transport chain
final common pathway that utilizes the harvested electrons from different fuels in the body; proton gradient it generates ultimately produces ATP
Aerobic metabolism / Oxidative phosphorylation
most efficient way of generating energy in living systems
laregely dependent on ADP/ATP and O2
mitochondrion
organelle that compleetes aerobic components of respiration
cristae
inner mitochondrial membrane is assembled into folds; maximize surface area; proton-motive force generated across it
proton-motive force
electrochemical proton gradient generated by the complexes of the electron transport chain essential for generating ATP
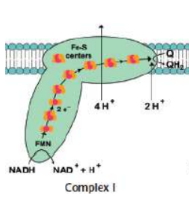
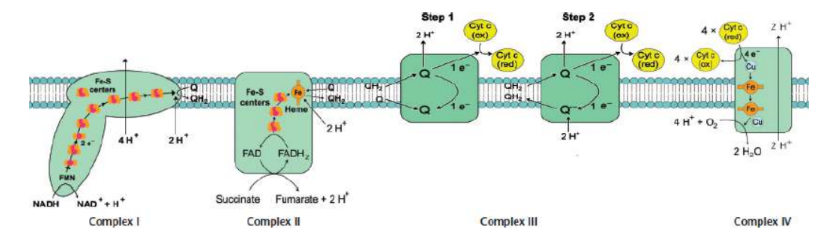
Complex I (NADH-CoQ oxidoreductase)
transfer of electrons from NADH to coenzyme Q (CoQ)
NADH transferring its electrons over to FMN → NAD+ and FMNH2
flavoprotein becomes reoxidized while the iron–sulfur subunit is reduced
reduced iron–sulfur subunit donates electrons to coQ → CoQH2
FOUR protons moved to intermembrane space
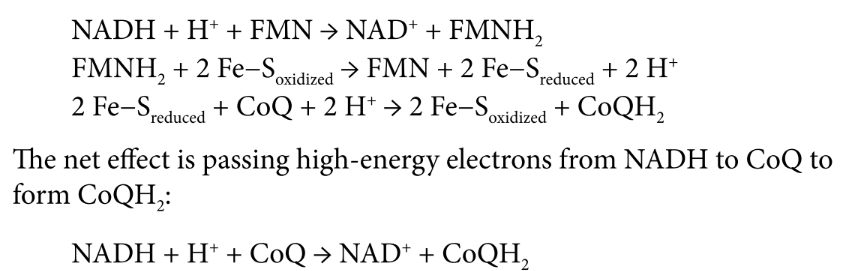
flavin mononucleotide (FMN)
coenzyme to flavoprotein in Complex I
coenzyme Q (ubiquinone)
recieves electrons from Complex I & II
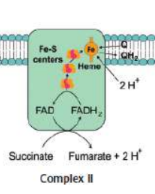
Complex II (Succinate-CoQ oxidoreductase)
transfers electrons from succinate to coenzyme Q
succinate from the citric acid cycle is oxidized by reducing FAD covalently bonded to the complex → fumarate and FADH2
FADH2 gets reoxidized to FAD as it reduces an iron–sulfur protein
reoxidizes the iron–sulfur protein as coenzyme Q is reduced
NO protons pumped
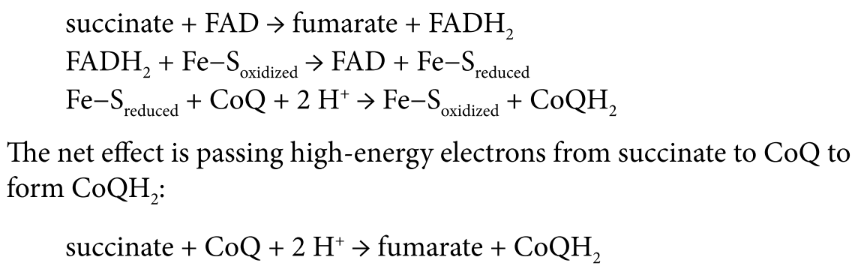
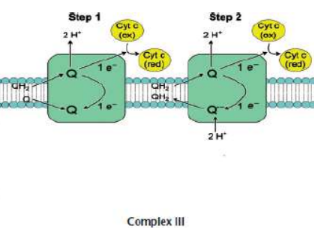
Complex III (CoQH2-cytochrome c oxidoreductase / cytochrome reductase)
transfer of electrons from coenzyme Q to cytochrome c via Q cycle
FOUR protons pumped

cytochromes
proteins with heme groups in which iron is reduced to Fe2+ and reoxidized to Fe3+
Q cycle
two electrons are shuttled from a molecule of ubiquinol (CoQH2) near the intermembrane space to a molecule of ubiquinone (CoQ) near the mitochondrial matrix
Another two electrons are attached to heme moieties, reducing two molecules of cytochrome c, assisted by iron-sulfur carrier
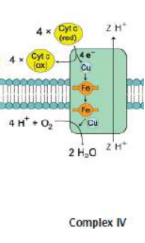
Complex IV (cytochrome c oxidase)
transfer of electrons from cytochrome c to oxygen, the final electron acceptor; includes subunits of cytochrome a, cytochrome a3, and Cu2+ ions
cytochrome oxidase gets oxidized as oxygen, becomes reduced, and forms water
TWO protons
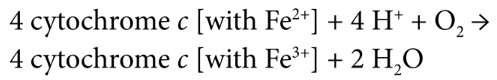
electrochemical gradient
gradient that has both chemical and electrostatic properties
shuttle mechanisms
transfers the high-energy electrons of NADH to a carrier that can cross the inner mitochondrial membrane from the cytosol; explains variation in amount of ATP in cells (30-32)
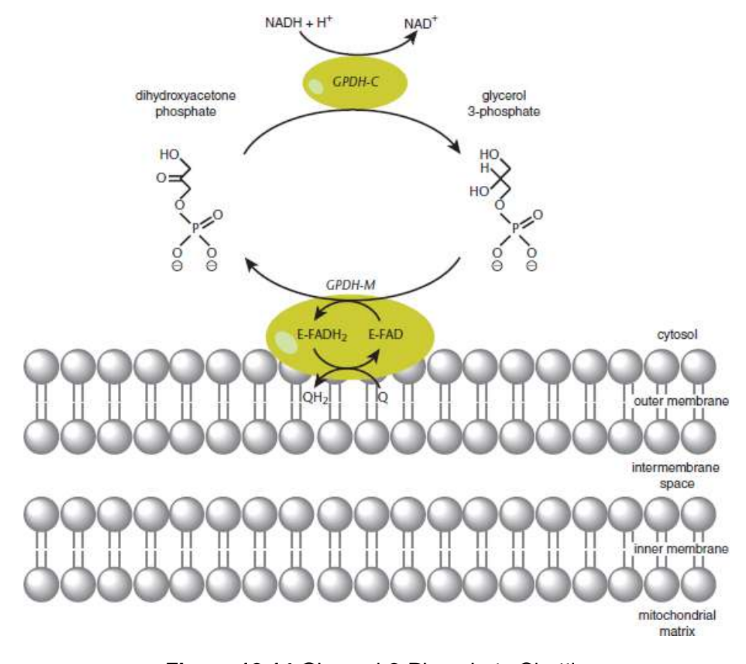
Glycerol 3-phosphate shuttle
cytosol contains one isoform of glycerol-3-phosphate dehydrogenase, which oxidizes cytosolic NADH to NAD+ while forming glycerol 3-phosphate from dihydroxyacetone phosphate (DHAP)
outer face of the inner mitochondrial membrane exists another isoform of glycerol-3-phosphate dehydrogenase that is FAD-dependent → reduced and transfers electrons to ETC via Complex II
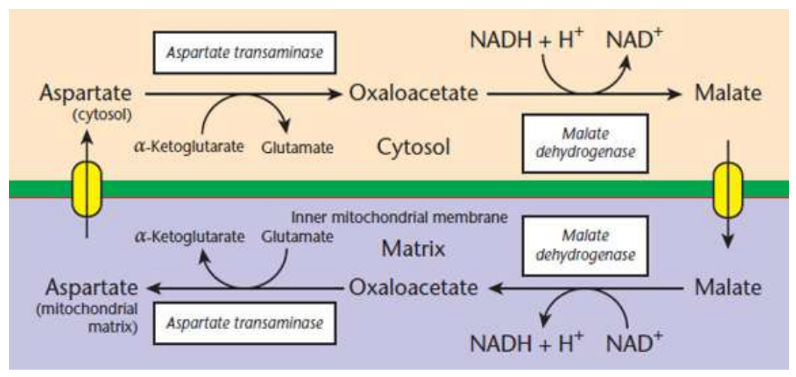
Malate–aspartate shuttle
Cytosolic oxaloacetate, which cannot pass through the inner mitochondrial membrane, is reduced to malate by cytosolic malate dehydrogenase → oxidation of cytosolic NADH to NAD+
mitochondrial malate dehydrogenase reverses the reaction to form mitochondrial NADH
pass along its electrons to the ETC via Complex I and generate 2.5 ATP per molecule of NADH
Recycling the malate requires oxidation to oxaloacetate, which can be transaminated to form aspartate, which crosses into the cytosol, and can be reconverted to oxaloacetate to restart the cycle
mitochondrial DNA
only encoded 13 of 100 polypeptides necessary for oxidative phosphorylation; mutation rate nearly ten times higher than that of nuclear DNA
chemiosmotic coupling
allows the chemical energy of the gradient to be harnessed as a means of phosphorylating ADP, thus forming ATP
ATP synthase
harness proton-motive force to form ATP from ADP and an inorganic phosphate
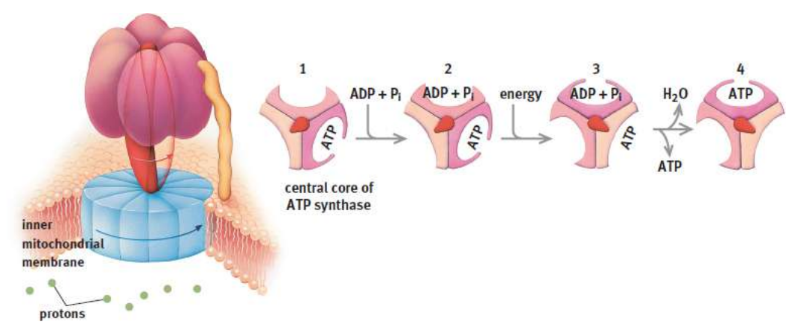
F0 portion (ATP synthase)
ion channel for protons to travel along gradient
ΔG∘ − 220 kJ mol
F1 portion (ATP synthase)
utilizes the energy released from this electrochemical gradient to phosphorylate ADP to ATP; reminiscent of a turbine, spinning within a stationary compartment to facilitate the harnessing of gradient energy for chemical bonding
conformational coupling
suggests that the relationship between the proton gradient and ATP synthesis is indirect; ATP is released by the synthase as a result of conformational change caused by the gradient
Uncouplers
compounds that prevent ATP synthesis without affecting the ETC, thus greatly decreasing the efficiency of the ETC/oxidative phosphorylation pathway; as ADP builds up and ATP synthesis decreases, the body responds to this perceived lack of energy by increasing O2 consumption and NADH oxidation; energy produced from the transport of electrons is released as heat
respiratory control
coordinated regulation of O2, ADP/ATP, NAD+/NADH, FAD/FADH2, citric acid pathways