SOM1 - Pharmaceutical suspensions
1/34
Earn XP
Description and Tags
Lecture 41-42
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
Dispersion of the solid phase creates a large interface (in a solid-liquid interface)
See diagram showing aggregation of particles to create a large interface to decrease surface tension, as the SA is the smallest in a single large particle

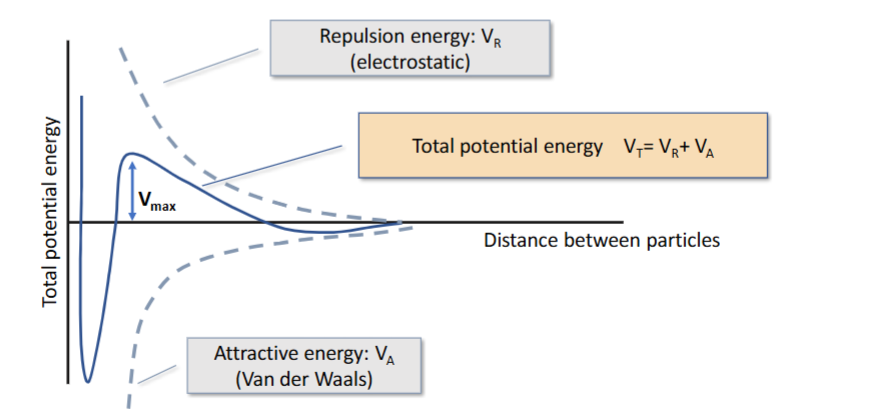
DLVO theory
Solid line in middle of graph is sum of repulsive and attractive energy
Below X axis means: overall attractive forces, primary minimum is caked solution, forces too strong to be undone by shaking, secondary minimum is flocculated , coalescence can be undone by shaking due to weak attractive forces
Above X axis means: Slightly above means particles stay suspended and don’t coalesce (stable suspension)
Uses of pharmaceutical suspensions
Pharmaceutical suspension can be used to:
Administer insoluble drugs
Minimise drug degradation (if it degrades in water)
Mask a bad taste
Alter absorption profile
Cater to special populations (children who can’t swallow pills, for example)
Advantages of suspensions
Easy dosage adjustment (just drink less/more)
Easy to administer, good adherence
Good bioavailability (in theory)
Nearly directly to absorption (doesn’t need to dissolve capsule shell, only needs to dissolve in body fluid)
Disadvantages of suspensions
Can be bulky to carry, due to extra liquid
May need refrigeration
Properties of the ideal suspension
Homogenous during dosing
Easy to resuspend just by shaking
Has an adequate viscosity
Characterised by a small particle size and narrow distribution
Because narrow distribution is equal dosage, and small size means faster dissolution in vivo
Why are suspensions prone to instability
Fine suspended particles
Small particles = high SA, and high instability as a result, as the system tries minimise the energy in the system
High surface energy
Thermodynamic instability
A high amount of free energy in a system will destabilise it.
Self-aggregation-settling-resuspension
Controlling these components allows the suspension to be resuspended with shaking
We can control sedimentation rate to achieve stability
What is settling + recite Stoke’s law
Downward movement of solid particles under the effect of gravity
Viscosity decreases settling, as viscosity prevent motion in the system
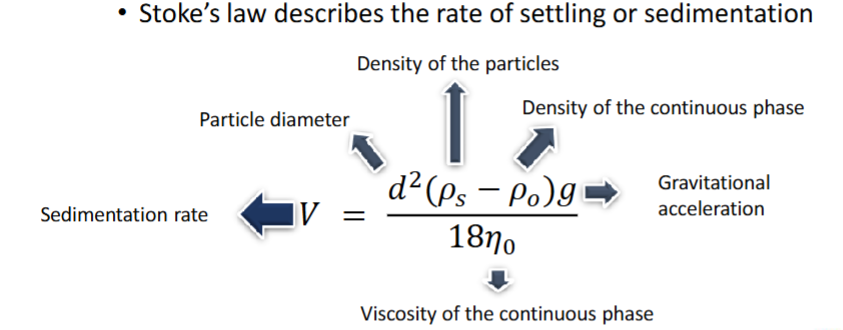
What effect does Stoke’s law apply to, and 5 requirements for it to be valid
Stoke’s law applies to settling
Only valid when:
Concentration of dispersed phase is <2% solids
This concentration is considered dilute
Dilute concentration means settling is not hindered, and so can occur freely, without turbulence
Dispersed phase is composed of spherical particles
Particle size distribution is narrow
Solvent is polar
Suspended particles are not subjected to Brownian motion
Brownian motion is the random movement of small particles, so the particles must be bigger than colloidal particles to not be affected

How can we prevent or reduce settling
Viscosity, particle diameter and density of dispersed/continuous phase are the factors we can change
Settling: Viscosity
Viscosity can be changed with suspending agents or viscosity enhancer
Downsides of excess viscosity
Oral
Can be hard to measure with a spoon or swallow
Injection
Can be painful
Topical
Can be gunky and hard to spread
What is the term used when the suspension aggregates at the primary minimum (DVLO)
Caking
What is the term used when the suspension aggregates at the secondary minimum (DVLO)
Flocculation
What implications does a high energy barrier have
Stable suspension, as the particles have high repulsive forces when closer together after the secondary minimum, so caking is very unlikely, as the particles need to overcome the energy barrier to get close enough to cake.
Aggregation at primary minimum
Initially, electrostatic forces (zeta forces) keep particles in suspension
Over time, uncontrolled settling occurs, leading to caking (very hard to redisperse by shaking alone)
When enough particles in the solution eventually settle, the pressure from the top of the settling layer allows the particles to break the energy barrier and overcome resistive forces, and a cake is formed
Supernatant = layer of liquid above sedimented layer
Turbid = opaque/cloudy (its turbid because the small particles sediment slower)

Aggregation at secondary minimum
Suspension is modified to reduce repulsive forces (compared to the caking suspension)
allowing it to avoid caking at primary minimum
But it does allow formation of loose aggregates (flocs)
Due to reduced repulsive forces, it also settles much quicker than a caking solution, but can be shaken to redisperse
Increases overall suspension volume, as the flocs stack with large gaps between

Properties of flocs
Weakly bonded aggregates
Quick settling rate due to a large particle size (stoke’s law)
Do not form cakes
Easily resuspended
Formed in the presence of floculating agents
How do electrolytes function as flocculating agents?
Reduced the repulsion barrier (Vr)
By neutralising the surface charge, repulsive forces are reduced, allowing for flocculation
Anionic agents are used to flocculate cationic particles (e.g. K₂SO₄)
Cationic agents are used to flocculate anionic particles (e.g. AlCl₃, NaCl)
How do polymers function as flocculating agents?
Polymers form a “bridge” between particles and allow them to get closer. Surfactants work in a similar way, and can be ionic or non-ionic. (Loose aggregate-forming agent pictured)
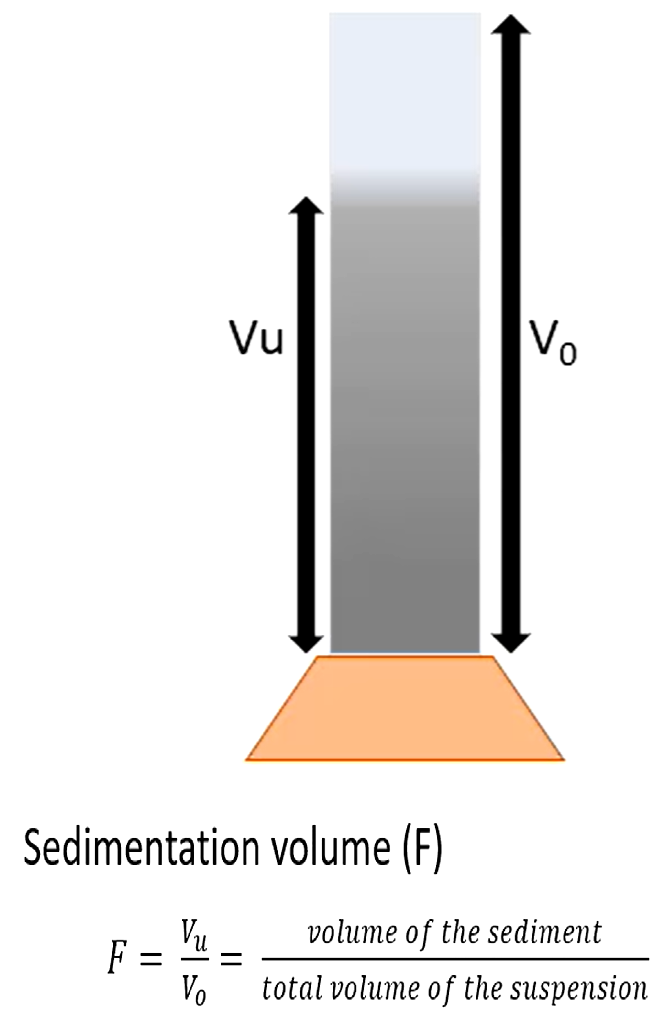
Efficacy of flocculating agents - calculating sedimentation volume
F will be higher on a flocculated suspension
Calculated by letting suspension sit for a moment with flocculating agent in a measuring cylinder, and then measuring sedimentation and total suspension volume.
Efficacy of flocculating agents - calculating degree of flocculation
β will be higher in a flocculated suspension
β indicates how good an agent is at flocculating a suspension (its basically a ratio between unaffected suspension and suspension with agent)
A functional flocculating agent will have a value higher than 1 (a value of 1 means it doesn’t do anything at all)

Controlled flocculation - describe what happens at positive, negative and neutral zeta potential
When zeta potential is too highly negative or positive, the particles repel each other, and don’t aggregate, until they sediment, at which point a cake is formed as they are rammed close together
At a weak/neutral zeta potential, the particles can aggregate gently with weak bonds and form flocs, so can be broken apart easily with shaking and don’t cake
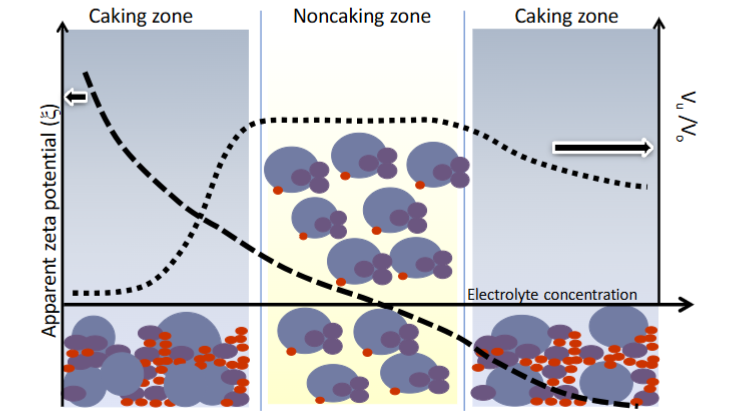
What is zeta potential
Effetively the “net surface charge” between the surface, slipping and stern layers. Measured at the slipping plane
What is Ostwald ripening?
When temperature fluctuations cause small particles to dissolve, and recrystallise onto larger particles.
Larger particles affect dispersed phase size, and therefore sedimentation rate, which could cause caking or grittiness
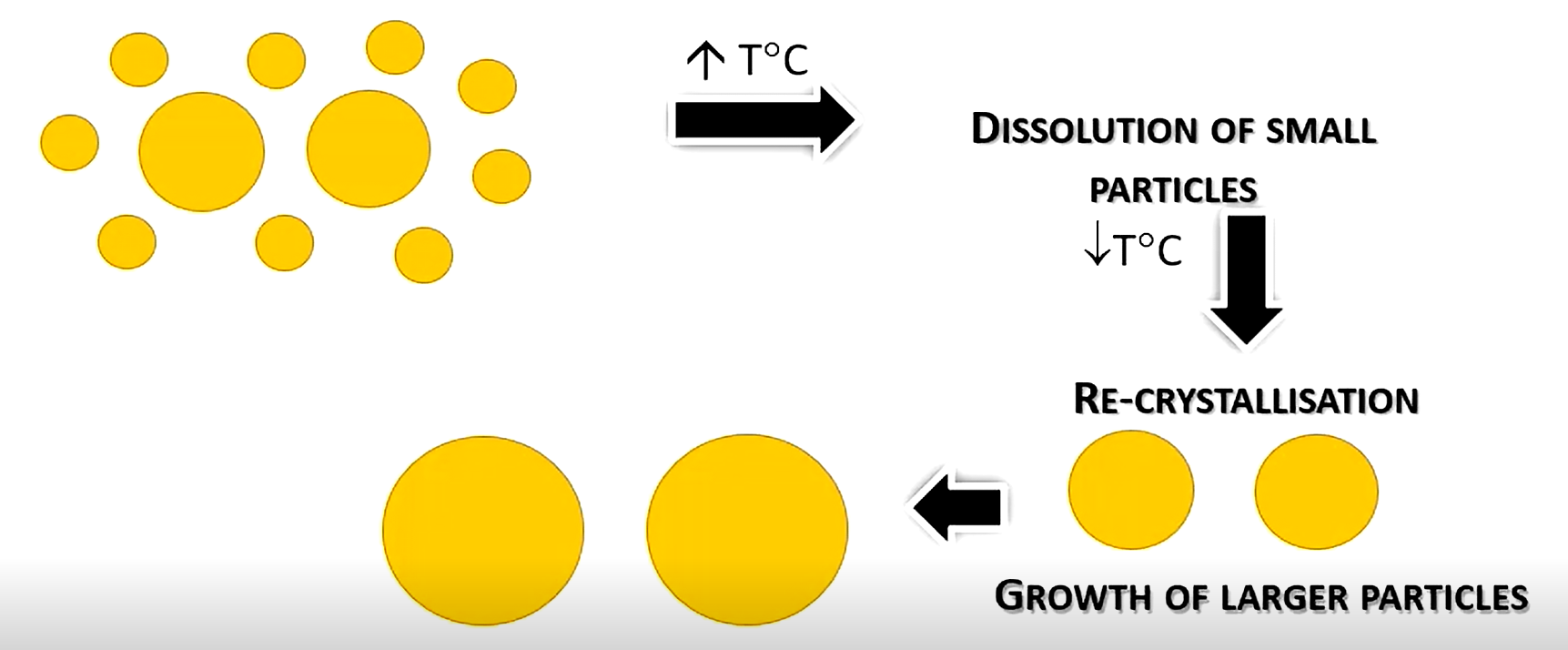
Why can surfactants worsen Ostwald ripening?
It can make smaller particles more soluble than larger particles, so more small particles dissolve quicker at more minor temperature changes.
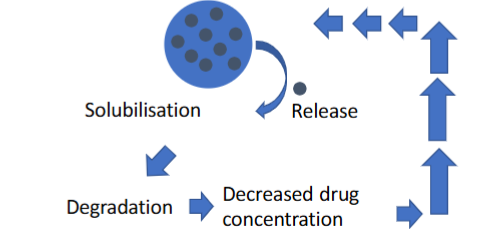
How do suspension solubility affect stability
Only the dissolved part of the suspension is prone to degradation, so the more soluble a suspension is, the more prone to degradation it is.
Often displays Zero-order degradation kinetics
Extemporaneous (unprepared) compounding
Take into account
Source of drug powder
(capsule, tablet, need to think about excipients)
Powder size
Reduce particle size (mortar and pestle usually)
Physio-chemical properties of dispersed phase
Determines
What excipients are required
How easily suspension is to prepare
Physical stability of preparation (in part)
Importance of particle size and size distribution
Affects:
Sedimentation
Stoke’s law
Ostwald ripening
Differences in solubility between small and large particles exaggerates ripening
Taste
perceived for particles >10mcm
Grittiness
Can be an irritant, perceived for particles >5mcm
Syringeability
size >25 mcm can be painful/too big to fit through needle tip
How do surfactants act as wetting agents
Surfactants bridge the gap between dispersed and dispersing phase, decreasing contact angle and interfacial tension.
How do low concentration hygroscopic solvents act as wetting agents + 2 examples
Coat the dispersed phase , decreasing contact angle
Glycerin, propylene glycol, ethanol
How do hydrophilic colloids act as wetting agents , and give 2 examples
Coat the dispersed particles in colloids, which increases affinity and decreases contact angle
Have no effect of interfacial tension, unlike surfactants
examples: bentonite, gums, cellulose derivatives and alginate
(most of these are also thickeners)
3 Concerns about surfactants as a wetting agent
Make sure it is anionic or non-ionic as cationic surfactants can be toxic
Bitter taste
Increase affinity for air, can cause foaming
Can exacerbate Ostwald ripening
What is a suspending agent?
Thickener, increases viscosity in a suspension, usually to decrease sedimentation rate
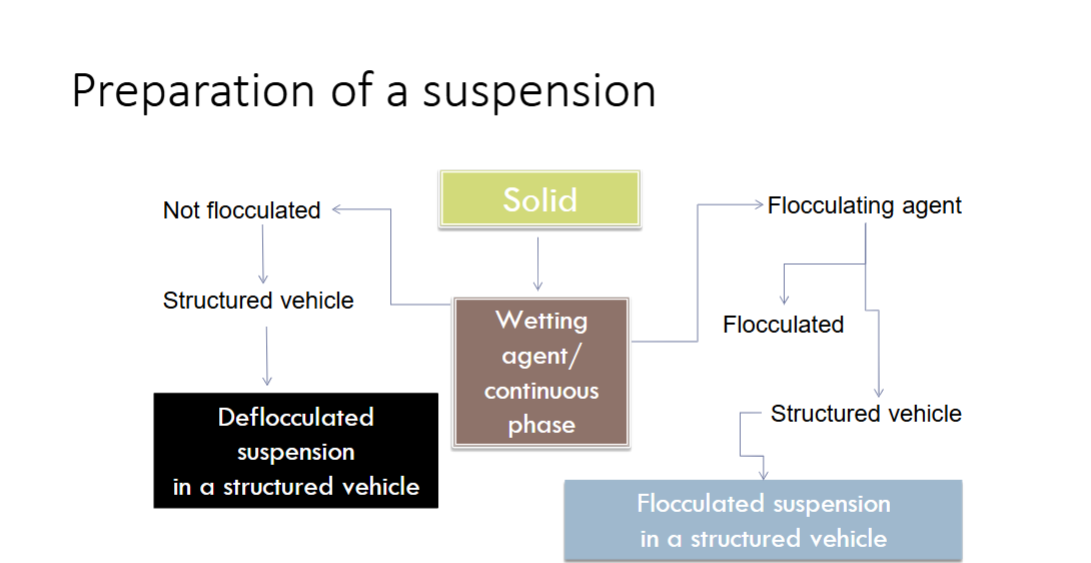
Preparation of a suspension flowchart
Quality attributes of suspensions
