Polymers and Composites
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
homopolymer vs copolymer
homo jus one type of building unit, co is two or more
Polymers: how are they 3D?
cross-linked between linear molecules to form 3D network
Polymers: may have ___ or __ regions, (i.e a varying % of each)
What determines physical properties?
amorphous or crystalline
Length/side branching/cross-linking
How does an increase in MW or chain length affect the polymer?
Increases rigidity, strength, melting temp
cross linking dramatically changes ___ of a polymer. How does an increase in cross-linking affect?
molecular weight
physical properties:
Increases rigidity and resistance to solvents
Increases glass transition temperature
Softening temperature from glassy solid to more flexible solid
Polymers elastic deformation: chains uncoil but do not slip past each other due to?
How well do they recoil when stress is removed?
crystalline regions, entanglements, cross-links
completely
Permanent deformation in polymer
What does the quantity of slippage depend on?
Polymer chains slide over one another and become relocated within material
Duration of loading
viscoelastic
combo of elastic and plastic strain with recovery of elastic strain that is time dependent
Thermoplastic
polymers that soften by heating and solidify on cooling, glass transition temperature
Poly (methyl methacrylate) and polystyrene are what kinds of plastic
thermoplastic
cross-linked poly(methyl methacrylate) and silicones are waht kind of plastic
thermosettng
thermosetting
polymers that solidify during fabrication but do not soften by heating
solvation properties are dependent on?
MW and crosslinking
Solvation properties;
Longer chains (or higher MW) dissolve ?
Why?
How does cross-linking affect dissolution?
small amount of swelling of dental polymers goes a long ?
what can this affect?
more slowly: due to polymers tend to absorb, swell and soften, then possibly dissolve in solvent
retards dissolution: highly cross linked cannot be dissolved
goes a long way: can affect fit of presthesis, some benefit in dentures as they shrink during processing
polymerization: definition and what are they two kinds?
chemical linking of monomer units to form high MW molecules: condensation and addition
Condensation polymerization, or ___: is what? What does this commonly make?
step growth
difunctional components, all become reactive simultaneously
Produce low MW byproduct (H20 or C-OH)
Impression materials (condensation sillicones, polysulfide)
addition polymerzation
starts from active center, adds monomer sequentially to form a chain
impression material and nylons are formed thru?
condensation polymerization
Addition polymerization can uses ____ polymerization. What are the stages?
free-radical
Induction: activation and induction
Propagation
Chain transfer
Termination
Free radical polymerization: Induction stage
Free radicals generated by activation of radical producing molecules using second chemical, heat, light, etc.
Free radical, (as part of the initiator), looks for something to attack, preferably double bond
Forms a bond with one carbon, leaving the other with excess electron (another free radical)
This initiates polymerization reaction
During free radical polymerization and during the induction phase, what molecule has the free radical initally?
Initiator
____ activates ___ __ initiator for denture base resins
____ activates __ in self cured resins
__ in some composite resins
Heat activates benzoyl peroxide
Chemical (tertiary amine) activates benzoyl peroxide
Light-activation
Free radical polymerization: Propogation phase?
Free radical reacts with another double bond, bonds to it, creates another free radical, and so on
Free radical polymerization: chain transfer
Active free radical of growing chain is transferred to another molecule (another monomer or inactivated polymer chain) and a new free radical for further growth is created
How does free radical polyermization end in the termination phase?
Additional typers of termination rxns may occur also, especially in ?
Coupling/Annihilation: free radical meets anther free radical
branched and cross linked polymers
PMMA: poly(Methyl methacrylate) is used for what? What can alter its properities?
denture bases
Pigmenting, composition, and processing variations
what is gutta percha used for?
What is it classified as?
fills space after root canal
natural polymer/plastic
Rubber
What is a rubber? Different kinds?
polymer of isoprene: may have different structural arrangements depending on where CH3 and H groups are (geometric isomer)
cis-isoprene: natural rubber
trans-isoprene: gutta percha
-(NH)-(C=O)-O-O structural unit:?
what used for?
polyurethane: orthodontic ligatures and modules or chains
polycarbonate is used for? What is it often reinforced with?
some plastic orthodontic brackets
ceramic fillers or glass fibers
Polyether is used for?
impression material
Polysulfide is used for?
impression material
Polyvinlyl siloxane is used for?
Impression material
composite definition
Dental composites?
mixture of two different classes of material
Mixture of polymer (resin) and ceramic (glass particles known as “fillers”)
the material of choice for direct esthetic anterior restorations?
Resin compsosite
What are the major components of the resin composite?
Matrix, filler, coupling agent
What is the matrix in the composite components?
What are the 3 common dimethacrylate matrices? (or can be blended)
plastic resin material that forms a continuous phase and binds the filer particles
BIS-GMA (propane), UDMA: urethane dimethacrylate, TEGDMA: triethylene glycol dimethacrylate
TEDGMA, HIDDMA (hexanediol dimethacrylate), DDDMA (dodecanediol dimethacrylate), PCDMA (polycarbonate dimethacryate): are all what?
These are sometimes called ___ bc they …
For example, TEDMA added varies with intended purpose: restorative resins are __wt% BIS-GMA, _%wt TEGDMA, while resin luting agents (which may need to flow better) may be a ? mixture
low viscosity monomers (matrix)
dilutents, lower viscosity of monomer system (compared to all BIS-GMA)
75%, 25%, 50-50
EBPADMA is?
newer dimetharcylaet: low water sorption substitute for BIS-GMA
Filler in a composite component is ?
What the the typical volume % or weight % of a composite?
reinforcing particles and/or fibers that are dispersed in the matrix
30-70% vol, 50-85% of weight
silica, borosillicate glass, lithium aluminum silicate, barium aluminum silicate, strontium or zinc glass are all examples of ?
Filler
Compared to the matrix itself, adding filler:
Flexure (2)?
Coefficient of thermal expansion?
Hardness?
Polymerization shrinkage?
Thermal conductivity?
increases flexure and compressive strength
Increases flexure and compressive modulus
reduces coefficient of thermal expansion
Increases hardness
Reduces polymerization shrinkage
Increases thermal conductivity (but not problematically so)
coupling agent does what?
When/How?
Most common:?
What would happen w out it?
bonds filler to matrix
surface of filler is treated with coupling agent before mixing with matrix
silanes
lack of bonding between filler and matrix, leading to decreases fracture resistance, fatigue resistance, and wear resistance
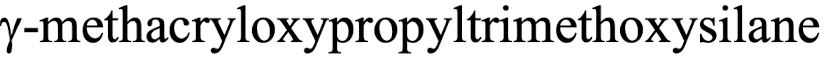
what is that?
typical silane: organic silicon compounds used as coupling agent
Composite additional components:
initiators, activators, accelerators?
Inhibitors?
Pigments?
Opacifiers?
Initiators: help begin polymerization process
Inhibitors: Prevent accidental/spontaneous polymerization
Pigments: Inorganic oxides
Opacifiers: Alter translucency
Titanium dioxide, aluminum oxide
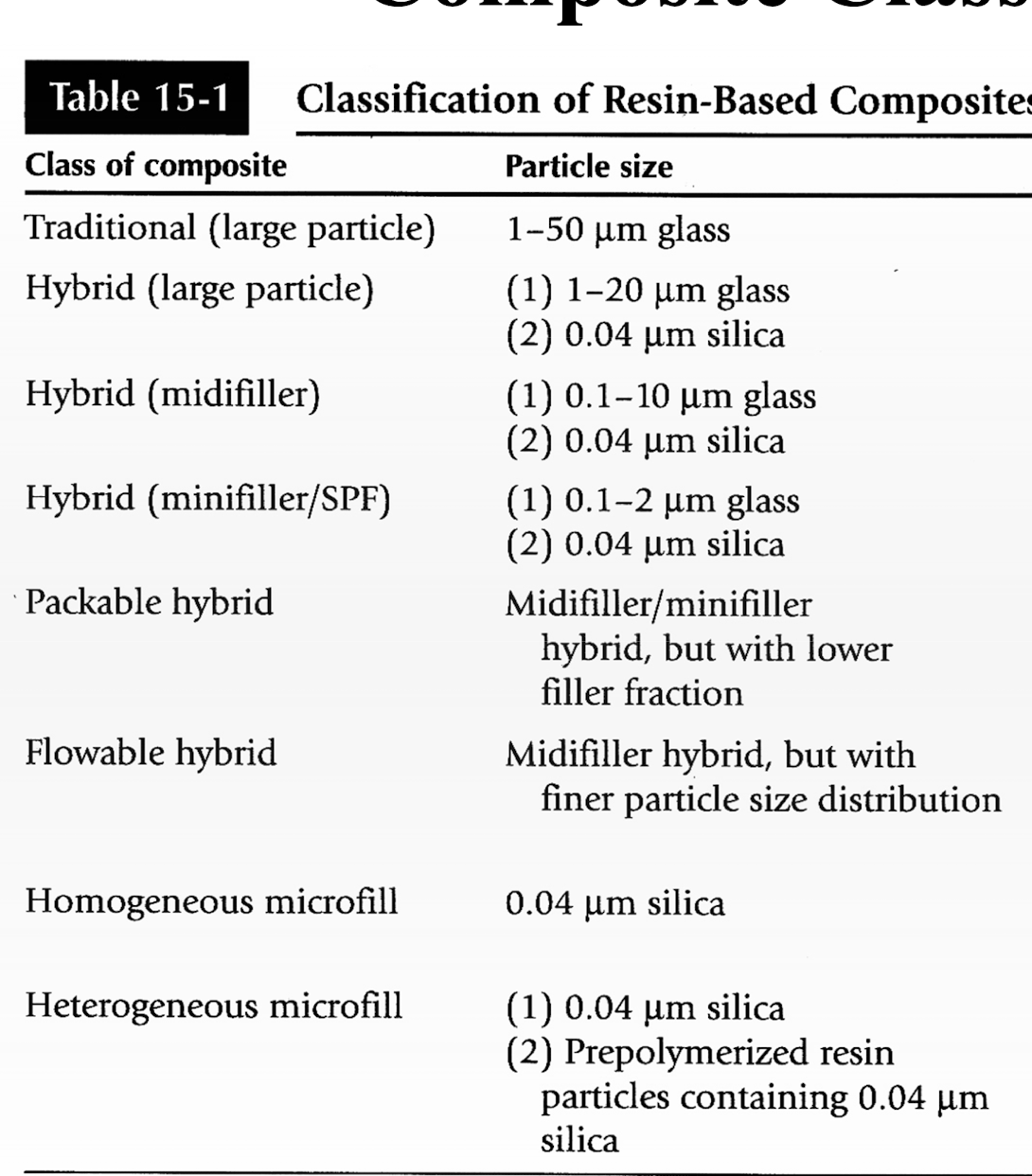
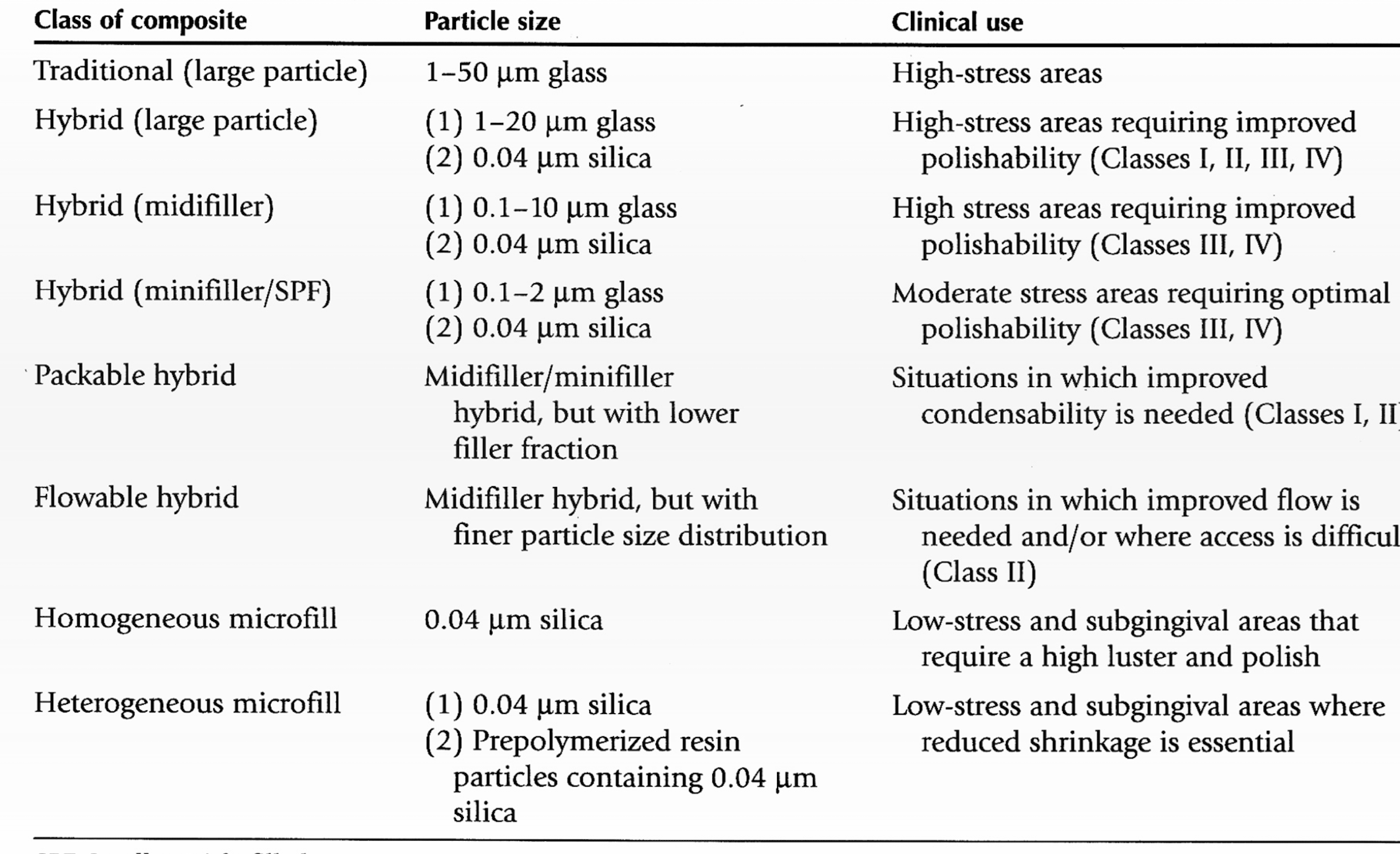
what are the different classifcations of composite? Which is arbitrary?
activation mode, usage, filler characteristics: arbitrary
Activation Mode: Composite Classification:
What are the different modes?
Light cured, chemically cured (AKA cold cured, self cured), dual cured
Composite classification: activation mode:
LIGHT CURED: what does it contain and what are the pros/cons
Contains photosensitive initiator (CQ, camphorquinone)
Pro: latitude in placement and time
cons: depth of cure → have to do it in increments
Composite classification: Chemically cured, or self cured, or cold cured:
What are the components? What is the pro/cons?
Base: contains activator (amine)
Catalyst: peroxide initiator
pro: proper mixing → uniform curing
con: limited working time, mixing porosity
Composite Classification: by usage
Anterior: superior surface texture and esthetic qualities
Posterior: higher resistance to wear and fracture
Universal: compromises between the two
Composite classification: filler characteristics
Based on? What are the types
particle size and distribution
All % are of filler!
Traditional:
60-70% vol
70-80% wt
10-100 um (biggest)
Hybrid
60-65% vol
75-90% wt
.01-0.1 and 1-50 um particle size
Microfilled
20-59% vol
35-67% wt
.01-0.1 um
Small
0.1-1 um
hybrid types of filler characteristics:
Generally contain: ?
What is the aim?
Microfill: 0.04 um
Second particle size: (large 1-20 um, mid: 0.1-10 um, small: 0.1 - 2 um
obtain smoothness of microfilled with higher mechanical properties of larger particle components
Hybrid variation: Packable vs. flowable
Packable
Higher filler % (48-67% vol, 65-81% wt)
Possibly fibers or textured filler surfaces
Used where condensability is needed
Flowable
lower filler % (30-55% vol, 40-60% wt)
Lower viscosity: but higher shrinkage, water sorption, and thermal expansion
Used where improved flow is needed or difficult to access
Match: Macrofill, microfill, hybrid, minifill/microhybrid, midfill
Better esthetically and polishable, but relatively weak
compromise, adequate strength but still esthetic
Strong but difficult to polish and keep smooth surface
Universals
Microfill
Hybrid
Macrofill
Minifill/microhybrid, Midifill
In general, mechanical properties of composite resin are related to?
How affect?
Filler content
More filler content:
Increased strength
Increased stiffness
Increased fracture toughness
increase contraction stress
less shrinkage
less absorption of water
Oxygen Inhibition Layer?
Signifcance?
In incured composite resin, atmospheric oxygen diffuses into resin. Oxygen inhibits polymerization by oxidizing free radicals into more stable peroxides with lower reactivity towards monomers
After light-curing, resin-rich, oxygen-inhibited layer is formed at the uppermost surface layer, approx 4-40 um thick
If small restorations arent finished, oxygen inhibition layer may be more susceptible to surface staining
Conflicting studies on if is important in the integrity of the incremental layers
Polymerization is seldom entirely complete in?
Degree of conversion?
Typical values? Does not imply?
What can leach out? Why is this an important consideration
Primarily in addition polymerization
% of C=C bonds converted to single bonds
50-70%, does not imply that 30-50% is not converted, as they can be incorporated into monomer, just not reacted
Residual monomer: potential allergen
Dental polymers ___ during curing monomer
shrink
Polymerization shrinkage:
What %?
What does this affect?
the greater amount of polymer present, the greater the potential for shrinkage upon polymerization
2-5% dependent on the class
bond of the composite to tooth, can lead to leakage and secondary caries
Contraction stress:
Greater contraction stress with?
In general?
greater degree of conversion
greater the filler content, the greater the contraction stress (even tho less shrinkage)
C factor
cavity configuration factor
ratio of bonded to unbonded surface of the composite
complex, but in general high C factors → high stress (more bonded surfaces, less unbonded surfaces, = higher stress)
Why care about contraction stress (6)
debonding, marginal staining, microleakage, secondary caries, enamel micro-cracks, post-operative sensitivity
Methods to lower contraction stress: formulation (not able to be controlled by clinician): 4
Larger monomers
non-bonded fillers
Adding inhibitors to slow curing
Different monomer systems
Clinical Factors to lower contraction stress
liners: use of GI, RMGI, or flowable composites
act as stress absorbers
Limiting constrains and curing in increments
Not just for depth reasons, maximizing free surfaces enhances stress relief by allowing more flow
Horizontal, vertical, oblique increments
Clinical factos to lower contraction stress: light cure protocols
What are the 3 kinds and whats the concept behind it?
Ramp: ramp irridance from low value to high value over 10 s
Step: irradiate at low intensity for 10 s then higher value
Pulse: short dose of low intensity, wait several mins, high intensity
Concept: Slow initial polymerization rxn, allowing more time for composite flow prior to gelation → reduces stress
Bisco, reflexions XLS dentin, Premise from Kerr, Kalore from GC america are what?
Low shrink composites
Do low shrink composites reduce shrinkage stress? What does it depend on?
most part: no
some lower cuspal deflection with Filtek LS and Kalore, but shrinkage stress still present.
Depends upon when shrinkage occurs (post gelation) and material properties like elastic modulus
Composite resin properties: Thermal properties
Coefficient of thermal expansion:
Composite > enamel > dentin
Affects margin => effects on bond integrity
Composite Resin properties: Water sorption and solubility
Dental composites ?
Some components of composites ? what does this affect? What kind of issues?
Absorb water: decreases with increased volume % of filler
Typical values: 0.3 - 2 mg/cm²
Some components of composites stablize
Typical 0.01-0.06 mg/cm²
Inadequately polymerized resin and/or silicon may leach out
Affects color stability and wear resistance
Residual monomer bicompatibility issuse
Composite wear forms
Abrasion
Generally 3 body (involving food/other particles)
More uniform loss of substance
Attrition
2 body, more localized
In high stress occlusal areas
Marginal Ditching
At restoration/tooth interface
Composite Wear: Corrosive wear?
Where issue and how?
Wear coupled with chemical attack
Issue especially in posterior restorations
Hydrolytic breakdown of resin
Breakdown of resin/filler interface
erosion of the surface from acids
How to mimic optimal, tooth like esthetics?
Layering technique
there may be a small shift in color with?
polyermization of resin composites
Amount depends on brand and shade: confirmation of shade with polymerized material
important to cure in increments why?
prevent uncured resin, limit stress
shade selection before or after rubber dam placement
before: dehydrated teeth elevate values
What are the four catergories of instruments of finishing and polishing
Coated abrasive disks and strips
Cutting carbide, diamond, and stones
Rubberized abrasives
Loose particular abrasives in the form of pastes and powders
Smooth highly polished surface has what effect?
What is the surface roughness threshold for plaque rentention?
Increases longevity
reduces plaque accumulation → less probability of gingival irritation or surface staining
0.2 um
Finishing vs Polishing
Finishing: gross contouring of restoration to obtain desired contour
Polishing: provides smoothness and reduction of finishing scratches
What is the main failure of composite resin?
0-5 years: primarily by restoration failure, then secondary caries
6-17 years: secondary caries
Bulk-fill compsite resin: what are the approaches
Placed in larger depth increments
Place in 1 layer
Most are a bottom bulk-fill layer and then capped with 2 mm increment of traditional composite
Bulk fill composite resin properties compared to traditional
tend to be more translucent: more light transmission → greater depth of cure, some have depth of cure > 4 mm
Tend to be less filled than traditional composite
Inferior mechanical properties: thats why capped for better strength and wear resistance
Indirect composite resin
Made in dental lab
Some are indicated for inlays, onlays, jacket crowns, veneers, crown and bridge
Cured with light-curing machine/box
CAD/CAM
composite resin blocks for milling
Indicated for inlays, onlays, veneers, and crowns
Bonded to tooth
Direct composite resin and MUSOD
TPH spectra (sim lab): universal compsoite: nanohybrid and microfiller compents
Surefil SDR flow (limited use in clinic): less filled and less strong but greater depth of cure
4 types of light-curing
Quartz-tungsten halogen
LED
Plasma arc (PAC)
Argon Laser
Differ in construction, light wavelength range generated, light intensity, cost, durability
____ have broad wavelength distributions
__ have more distinct and narrower wavelength distributions depending on chips
Quartz-tungsten-halogen and PAC LCUs
LEDs
Most compsostie resins (>90%) contain __ as a photoinitator and _ as activator (which)
CQ (camphoquinone),, amine (DMAEMA)
Photoinitators will have certain __ for excitation/absorption
The spectral emission from light curing unit should overlap the ?
wavelength
absorption spectrum of photoinitator in composite
Total energy of the irradation or radiant exposure = ?
Exposure reciprocity law?
What does it influence? How much is needed?
Irradiance * irradation time
Influences degree of conversion and mechanical properties: 4000-16000 mW*sec/cm²
Law: Comparable material properties will result as long as the same radiant exposure is obtained (studies disagree)
Irradiance variability: Should tip be moved around?
Light exiting curing units is not necessarily uniform
No: but if using circular motions, increase curing time so that overall time is greater at single point
Light attenuation
Light intensity decreases with?
During light curing, it may be difficult to have light tip directly adjacent to tooth
Cavity/prep may increase distance from light tip to material
Decreases with distance
Depth of cure:
Light penetration is ___ as it passes through material
Lower depths? Darker shades?
Attenuated
Lower depths do not feel same light intensity: cure in 2 mm or less increments
Darker shades attenuate light to greater extent
angle of light curing units
Light tip as close as possible (w out touching) and parallel to surface
45 degree angle significantly reduces energy delivery
High levels of blue light?
Greatest damage at?
immediate irreversible, chronic exposure accelerated retinal aging and degeneration
440 nm
Does composite resin sufficiently bond to tooth structure?
NO: bonding and bonding agents