CVS
1/124
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
125 Terms
What is the difference between primary and secondary hypertension (HTN)?
Primary hypertension (90%) - has no identifiable cause.
Secondary hypertension (10%) - has a known underlying cause.
Give examples of causes of secondary hypertension?
“ROPED” mnemonic:
R – Renal disease
O – Obesity
P – Pregnancy-induced hypertension or pre-eclampsia
E – Endocrine e.g., hyperaldosteronism
D – Drugs (e.g., alcohol, steroids, NSAIDs, oestrogen and liquorice)
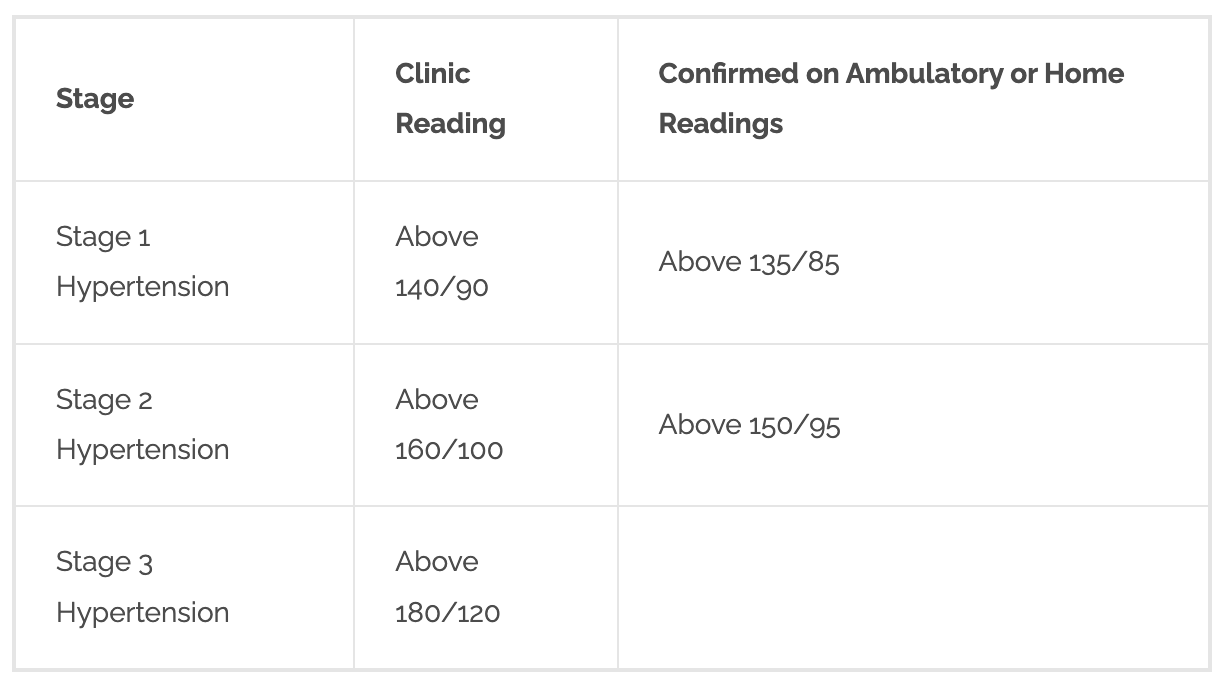
How is HTN classified?
Stage 1 hypertension — clinic blood pressure ranging from 140/90 mmHg to 159/99 mmHg and subsequent ABPM daytime average or HBPM average blood pressure ranging from 135/85 mmHg to 149/94 mmHg.
Stage 2 hypertension — clinic blood pressure of 160/100 mmHg or higher but less than 180/120 mmHg and subsequent ABPM daytime average or HBPM average blood pressure of 150/95 mmHg or higher.
Stage 3 or severe hypertension — clinic systolic blood pressure of 180 mmHg or higher or clinic diastolic blood pressure of 120 mmHg or higher.
What is accelerated (or malignant) HTN?
A severe increase in blood pressure to 180/120 mmHg or higher (and often over 220/120 mmHg):
with signs of retinal haemorrhage and/or papilloedema (swelling of the optic nerve).
Who should receive a same-day specialist assessment due to HTN?
For people with:
Clinic BP of 180/120 mmHg and higher:
With signs of retinal haemorrhage or papilloedema (accelerated hypertension) or life-threatening symptoms, such as new onset confusion, chest pain, signs of heart failure, AKI.
Suspected phaeochromocytoma (adrenal medulla tumour), for example headache, palpitations, pallor, abdominal pain.
What does NICE recommend all patients with a new diagnosis of HTN to have?
To assess for target organ damage:
Urine albumin:creatinine ratio - for proteinuria and dipstick for microscopic haematuria to assess for kidney damage
Electrolytes, creatinine, and eGFR - for CKD.
Bloods - for HbA1c, renal function and lipids
Fundus examination - for hypertensive retinopathy
ECG - for cardiac abnormalities, including left ventricular hypertrophy.
Calculating the QRISK score - offer statin if above 10%.
Lifestyle advice for HTN?
Healthy diet, stopping smoking, reducing alcohol, caffeine and salt intake and taking regular exercise.
How is HTN treated?
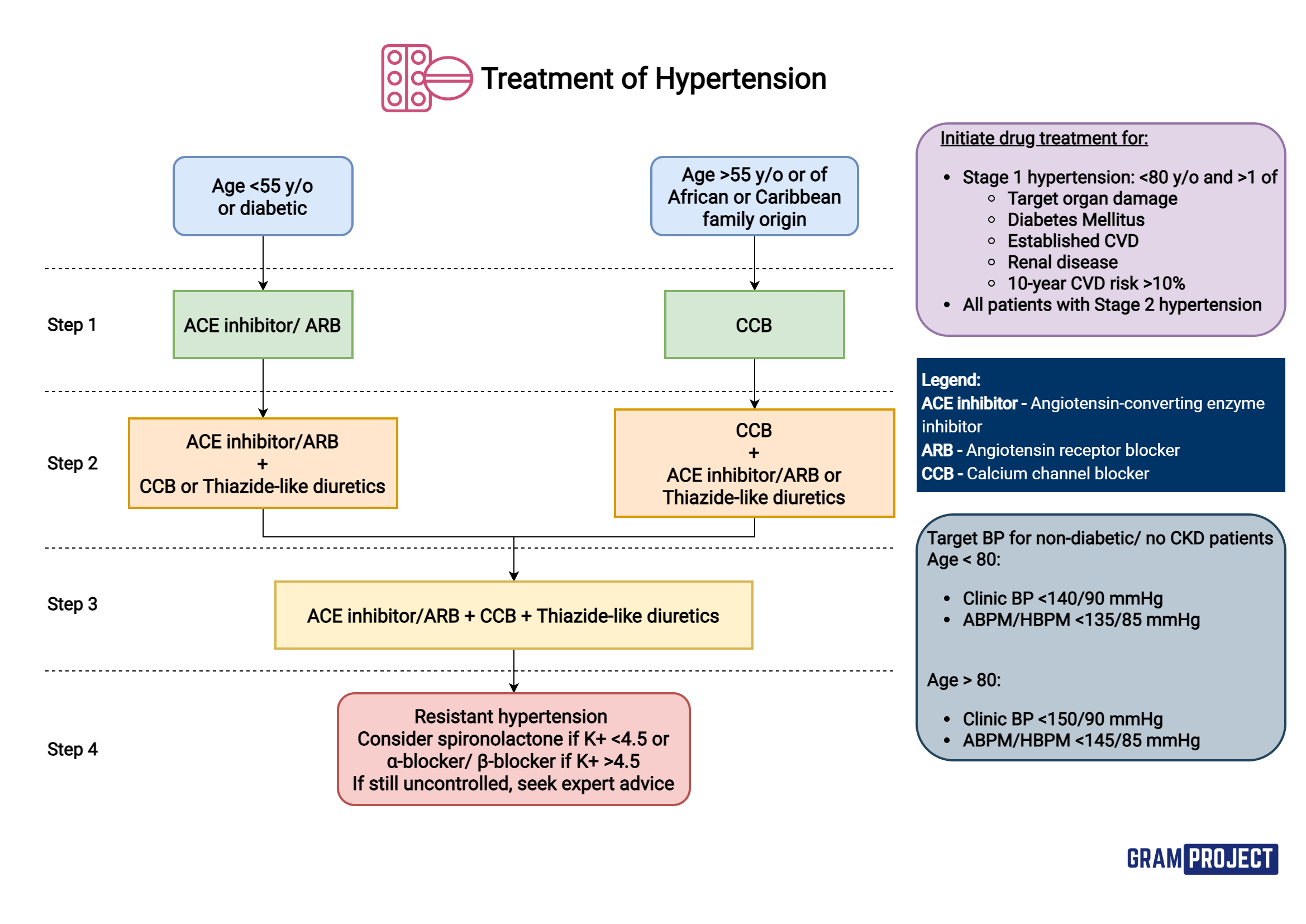
Black African or African-Caribbean family origin - prefer ARB
ACE inhibitors and ARBs - do not use in women who are pregnant, planning pregnancy, or breastfeeding.
Do not combine an ACE inhibitor with an ARB for HTN.
Thiazide-like diuretics - alt if CCB not tolerated - commonly due to ankle oedema.
What are target clinic blood pressures based on age?
Age under 80 years — clinic blood pressure below 140/90 mmHg; ABPM/HBPM below 135/85 mmHg.
Age 80 years and older — clinic blood pressure below 150/90 mmHg; ABPM/HBPM below 145/85 mmHg.
How can spironolactone be used used for HTN?
Spironolactone is a potassium-sparing diuretic.
MoA: blocks action of aldosterone in the kidneys = sodium excretion and potassium reabsorption.
helpful when thiazide diuretics are causing hypokalaemia.
Caution: increases risk of hyperkalaemia.
ACE inhibitors can also cause hyperkalaemia.
angiotensin II promotes aldosterone release → so if ACE blocked → less angiotensin II made = less aldosterone. (RAAS inhibited)
Thiazide-like diuretics can also cause electrolyte disturbances.
So must monitor U+Es regularly with these drugs.
How to treat HTN in pregnancy?
First line = labetolol.
2nd line = nifedipine modified-release
3rd line = methyldopa (needs to be stopped within two days of birth)
MoA of ACE inhibitors
Inhibit angiotensin converting enzyme (ACE).
Blocks conversion of angiotensin I to vasoconstrictor angiotensin II = leading to vasodilation and reduced BP.
Blocks kininase production, ∴ prevent breakdown of bradykinin = bradykinin accumulated = has vasodilator effects.
Bradykinin - may cause ACEi-induced cough (give ARB instead).
Cautions/adverse effects for ACE inhibitors
Dry irritant cough
Angioedema - more common in black patients (give ARB instead).
due to bradykinin accumulation - vasodilation - fluid leakage into tissues = swelling
In severe cases - swelling of pharyngolaryngeal - threatens airways.
Hyperkalaemia (avoid potassium supplements + potassium-sparing diuretics).
Concomitant NSAID use (renal damage risk) + potassium-sparing diuretics (hyperkalaemia).
First dose hypotension
Contraindications for ACE/ARB inhibitors
- Pregnancy + breastfeeding.
- Renovascular disease.
ARB + ACE = CI
Monitoring for ACE inhibitors
- Can give low dose spironolactone (for severe HF) with ACEi - monitor serum potassium.
- Renal function + electrolytes.
- May cause renal impairment - but is renal protective in diabetics.
Common examples of ACE inhibitors?
(-‘pril’) - ramipril, lisinopril, enalapril, perindopril etc.
MoA of angiotensin receptor blockers (ARBs)
- Block the action of angiotensin II at the angiotensin II AT1-receptor
- Angiotensin II = vasoconstrictor + stimulates aldosterone release.
- ∴ antagonism = vasodilation + potassium retention (plus salt + water excretion).
Caution: hyperkalaemia + impaired renal function
MoA of calcium channel blockers (CCBs)?
decrease calcium influx into vascular smooth muscle cells.
by inhibiting the voltage-gated calcium channels in the cell membrane.
this promotes vasodilation and has negative inotropic and chronotropic effects
negative inotropic = decrease force of contractility
negative chronotropic = decrease HR effects
Dihydropyridine group:
- Work almost exclusively on the l-type calcium channels in the peripheral arterioles.
- Reduce BP by reducing total peripheral resistance.
Verapamil + diltiazem (rate-limiting):
- Primarily on the heart - reducing HR + cardiac output (CO).
When to avoid CCBs?
Calcium-channel blockers, with the exception of amlodipine, should be avoided in heart failure.
Adverse effects of CCBs?
- Constipation - verapamil.
- Hypotension
- Oedema, flushing, headache - due to peripheral vasodilation
- Gum hypertrophy - dihydropyridines.
Monitoring for CCBs?
- Rate-limiting overdose - cardiac depressant effect - causing hypotension + arrhythmias incl. complete heart block + asystole.
- Sudden withdrawal - some evidence of exacerbation of myocardial ischaemia.
Contraindicated in pregnancy + breast feeding.
Benefits of the different types of CCBs?
Dihydropyridine examples (-‘pine’) = amlodipine, felodipine, lacidipine, lercanidipine, nicardipine, nifedipine.
- Have no anti-arrhythmic activity.
- Rarely cause/worsen heart failure.
Long acting dihydropyridines (once-daily) preferred:
- More convenient for pt’s.
- Avoid large fluctuations in plasma drug concentrations that may be associated with adverse effects.
MoA of diuretics?
- Blocks distal renal tubular sodium reabsorption in the nephron.
- Initially - reduce BP by sodium excretion and reducing circulating blood volume.
- Long-term - reduce total peripheral resistance (suggests direct vasodilatory action).
Caution: risk of hypokalaemia - due to urinary potassium loss.
Types of diuretics?
- Thiazide diuretic examples = bendroflumethiazide, hydrochlorothiazide.
- Thiazide-like diuretic (T-LD) examples = chlortalidone, indapamide.
How are diuretics administered?
Administered start of day so diuresis does not interfere with sleep.
Although spironolactone is not a first-line Tx for HTN, when could it be used?
Spironolactone (aldosterone antagonist) - not suitable first-line therapy - but is an increasingly important option at low dose (25 mg daily) for resistant hypertension.
- Suspected hyperaldosteronism - spironolactone may prove to be effective.
MoA of alpha-blockers (α-adrenoreceptor blockers)?
- Antagonise α-adrenoreceptors in the blood vessel wall
- ∴ prevent noradrenaline induced vasoconstriction - as noradrenaline cannot bind = vasodilation.
- ∴ reducing total peripheral resistance + BP.
Examples of alpha-blockers (α-adrenoreceptor blockers)?
- Prazosin dis- = short-acting + causing first-dose hypotension.
- Newer agents e.g., doxazosin + terazosin - longer duration of action.
CI = urinary incontinence
MoA of beta-blockers (BBs) - β-adrenoreceptor antagonists?
Uncertain in HTN.
Antagonise effects of sympathetic nerve stimulation or circulating catecholamines at beta-adrenoceptors.
Beta1-receptors - predominant in the heart (and kidney).
Beta2- receptors are predominant in other organs such as the lung, peripheral blood vessels and skeletal muscle.
Heart: Blockade of beta1-receptors in the sino-atrial node reduces heart rate (negative chronotropic effect) and blockade of beta1-receptors in the myocardium decrease cardiac contractility (negative inotropic effect)
Kidney: Blockade of beta1-receptors inhibit the release of renin from juxtaglomerular cells and thereby reduce the activity of the renin-angiotensinaldosterone system.
Central and peripheral nervous system: Blockade of beta-receptors in the brainstem and of prejunctional beta-receptors in the periphery inhibits the release of neurotransmitters and decreases sympathetic nervous system activity.
What are the types of beta-blockers?
Non-selective β-blockers - ADRs due to antagonism of β2-adrenoceptors = asthma and worsened intermittent claudication (muscle pain in the legs when active + stops when at rest).
Cardio selective (β1-selective) β-blockers - not entirely free of these ADRs.
Patients who develop very marked bradycardia and tiredness may tolerate a drug with partial agonist activity such as pindolol.
E.g., bisoprolol, nebivolol, atenolol, propranolol, metoprolol.
Contraindications for BBs?
- Asthma/COPD.
- Heart block.
MoA of methyldopa and moxonidine?
Inhibit sympathetic outlow from the brain = reduction in total peripheral resistance.
Methyldopa is not widely used - causes central adverse effects, including tiredness and depression.
used in pregnancy because it does not cause foetal abnormalities.
Common/significant side effects of antihypertensives?
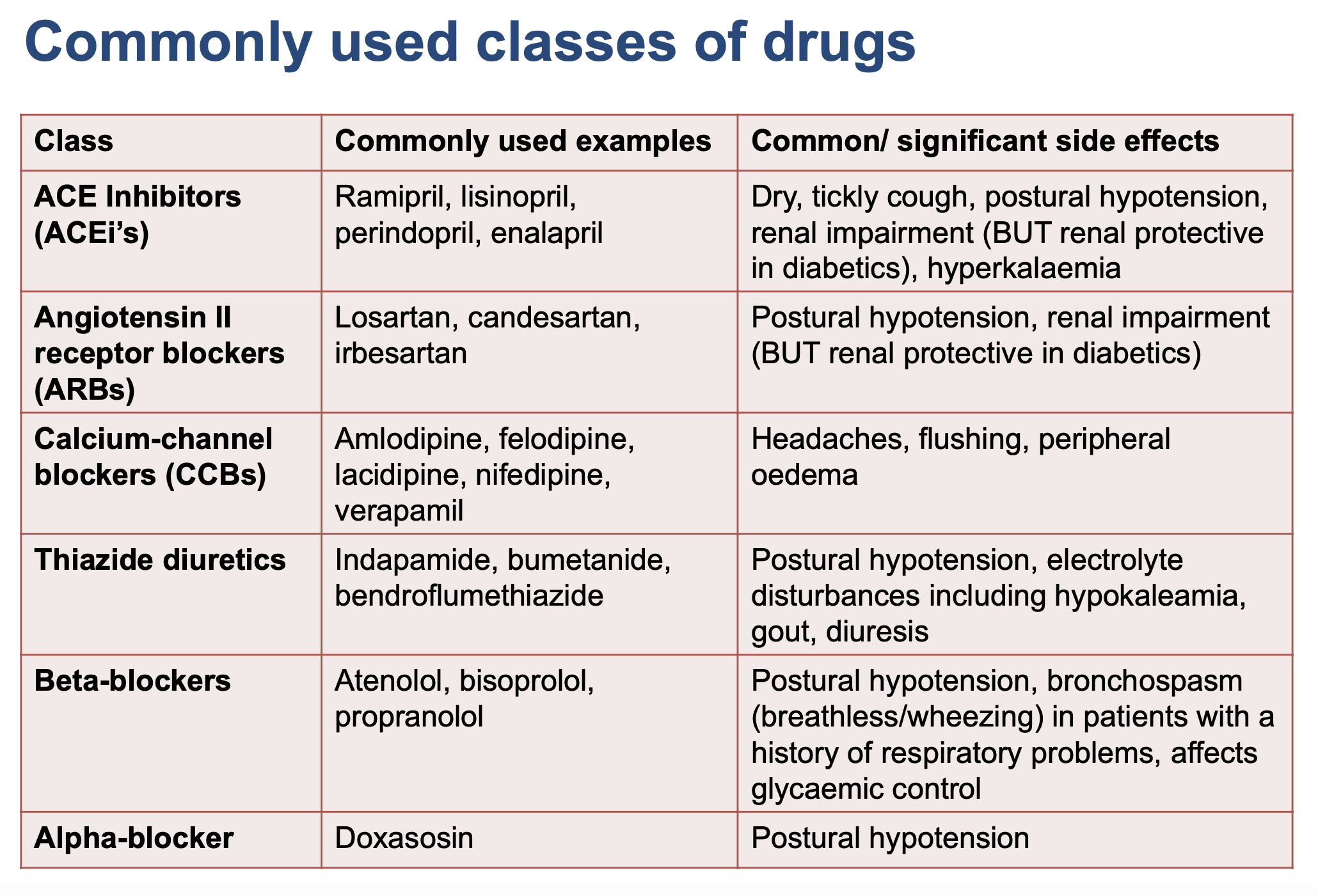
CCBs + grapefruit juice
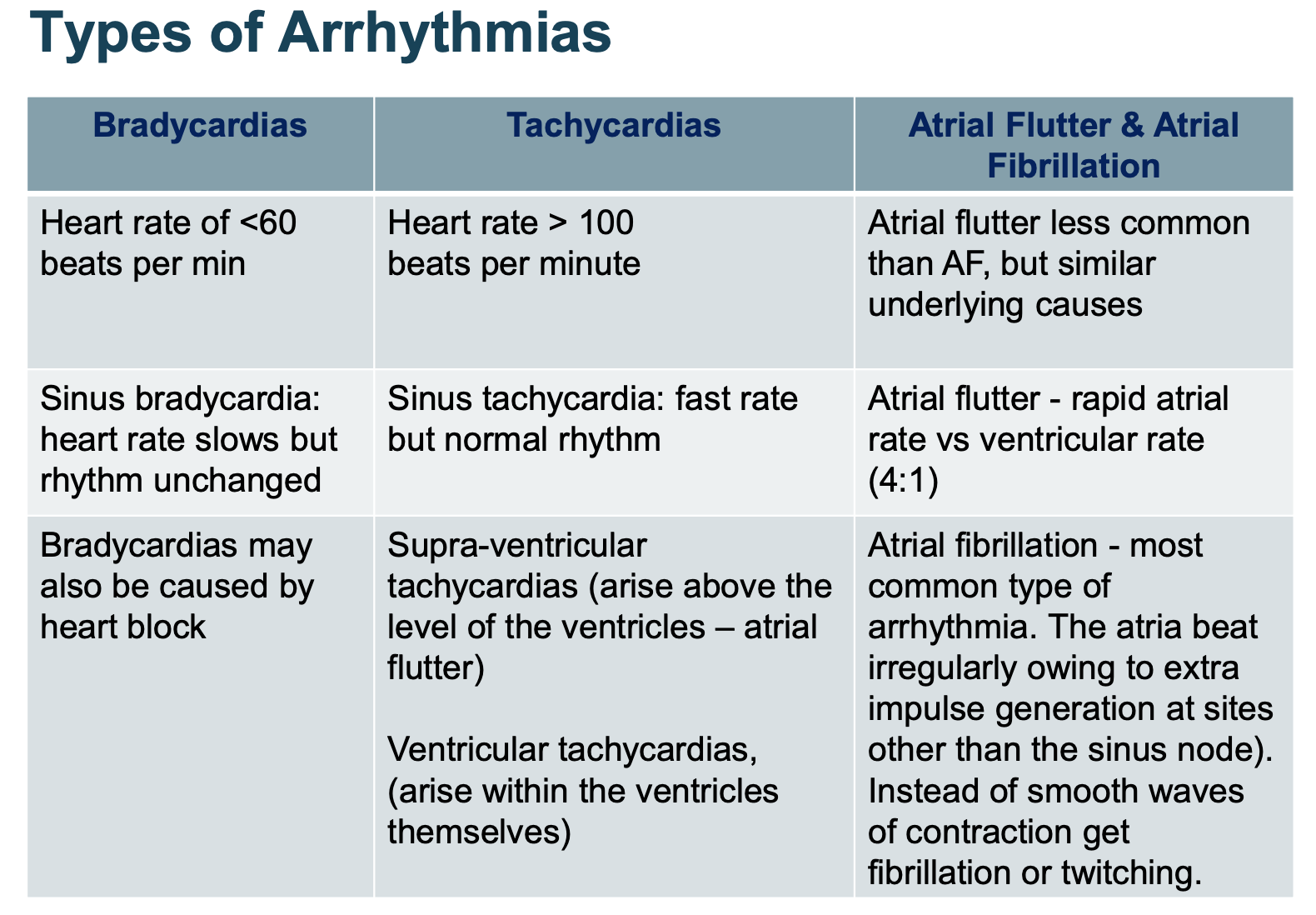
What is an arrythmia?
Any change in normal rate/rhythm of the heart.
result from an interruption to the normal electrical signals that coordinate the contraction of the heart muscle.
How does an arrhythmia occur?
Develop by:
Altered impulse generation
Change in ability of heart to generate electrical impulses spontaneously – leads to depolarisation of pacemaker cells in sinus node
Or through AP generation from a site other than sinus node
Altered impulse conduction
Complete/partial block of conduction pathways within the heart
Types of arrythmias?
Where does a heartbeat begin? Where does it continue?
Begins in sinoatrial node (SA) = programmed to regularly trigger heartbeats (are pacemakers)
SA = specialised conductive bundles which ensure contraction is coordinated + chronological
SA node --> (both) atrial muscles contract --> atrioventricular (AV) bundle/node contracts --> (both) ventricular muscles contract
Delays = properties of conduction of the signals along the fibres
Explain properties of ECG/EKG tracks:
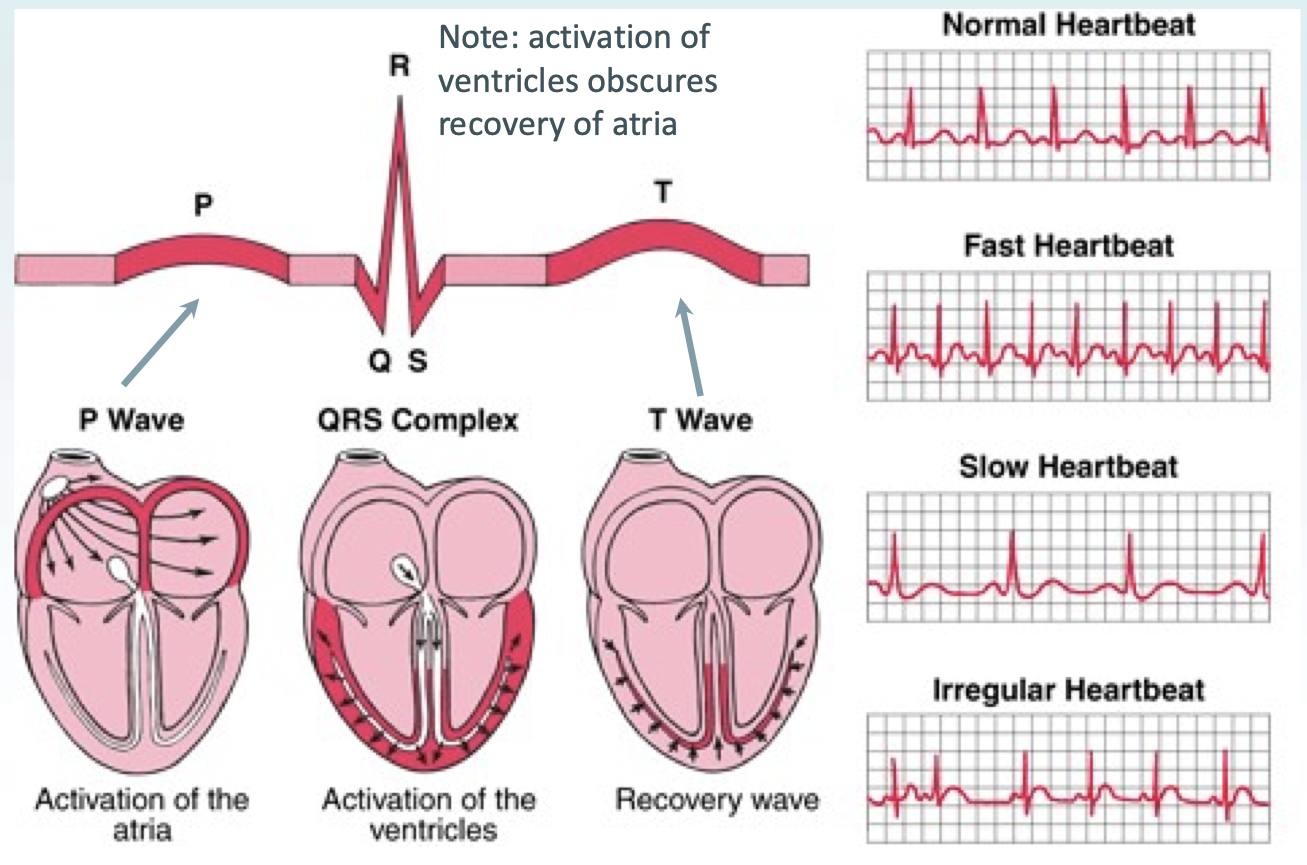
P wave = activation of atria
QRS complex = activation of ventricles
T wave = recovery wave
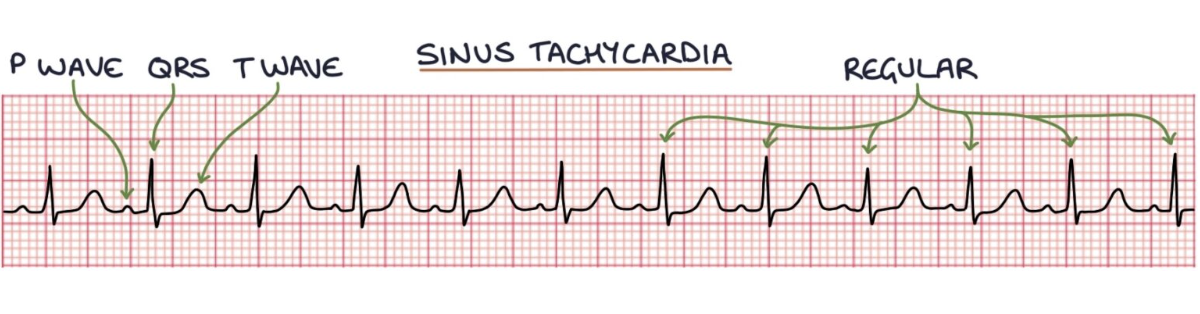
How does a sinus tachycardia and supraventricular tachycardia present on graph?
Sinus tachycardia will take the normal P wave, QRS complex and T wave pattern.
not an arrhythmia and is usually a response to an underlying cause, such as sepsis or pain.
Supraventricular tachycardia (SVT) looks like a QRS complex followed immediately by a T wave, then a QRS complex, then a T wave, and so on. There are P waves, but they are often buried in the T waves, so you cannot see them.
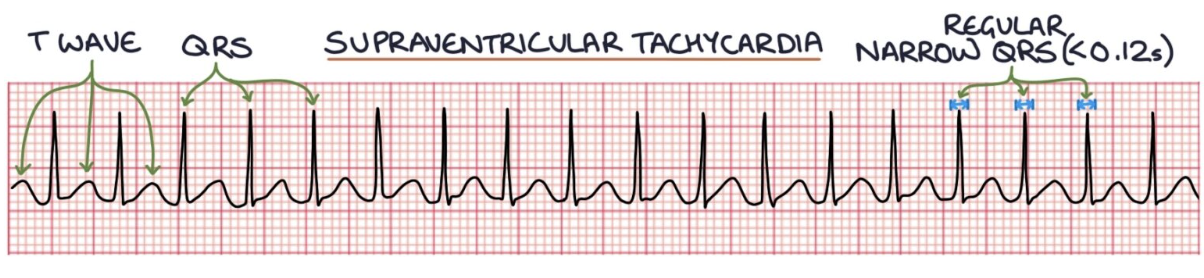
SVT - treated with sotalol.
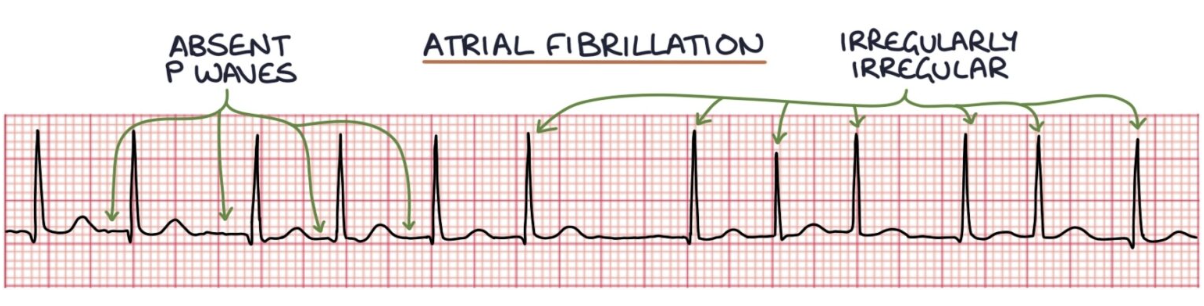
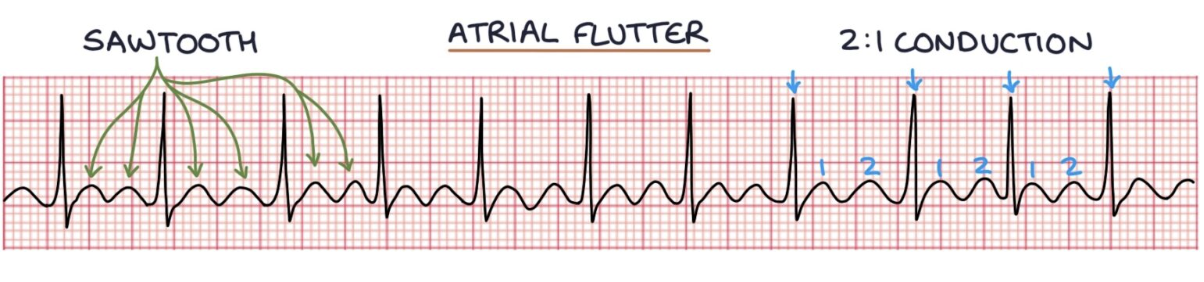
How does atrial fibrillation and atrial flutter present on graph?
Atrial fibrillation can be identified on an ECG by absent P waves and an irregularly irregular ventricular rhythm.
Atrial flutter - atrial rate is ~300 bpm and gives a saw-tooth pattern on the ECG.
A QRS complex occurs at regular intervals depending on how often there is conduction from the atria.
often results in two atrial contractions for every one ventricular contraction, giving a ventricular rate of 150 bpm.
What is atrial fibrillation (AF)?
i.e., electrical activity in the atria is disorganised = rapid atria contraction - uncoordinated and chaotic.
= absence of the P-waves on an ECG in atrial fibrillation.
electrical signal that passes through the atrioventricular node to the ventricles is random and often rapid = irregularly irregular ventricular contractions.
on an ECG - QRS complexes are irregularly irregular, meaning that they occur randomly without a predictable pattern.
How is AF classified?
Classified according to the pattern, clinical presentation, duration, and spontaneous termination of episodes into:
Paroxysmal (episodes terminate within 7 days of onset).
Persistent (lasts longer than 7 days).
Longstanding persistent (duration at least 12 months).
Permanent (no further attempts to restore or maintain sinus rhythm).
Most common causes of AF?
“SMITH” mnemonic:
S – Sepsis
M – Mitral valve pathology (stenosis or regurgitation)
I – Ischaemic heart disease
T – Thyrotoxicosis
H – Hypertension
Alcohol and obesity are lifestyle causes.
How does AF present?
Often asymptomatic, and is an incidental finding. May be diagnosed after a stroke.
Patients may present with:
An irregular pulse with or without symptoms, such as:
Palpitations
Shortness of breath
Chest pain/tightness
Dizziness or syncope (loss of consciousness)
Symptoms of associated conditions (e.g., stroke, sepsis or thyrotoxicosis)
What to investigate in AF?
Things to investigate in those with AF:
Full blood count
Detect anaemia + sepsis evidence
Thyroid function tests
Hyperthyroidism may cause AF
Blood urea, electrolytes, calcium, glucose
Electrolyte disturbances may precipitate AF
How to manage new-onset or acute AF?
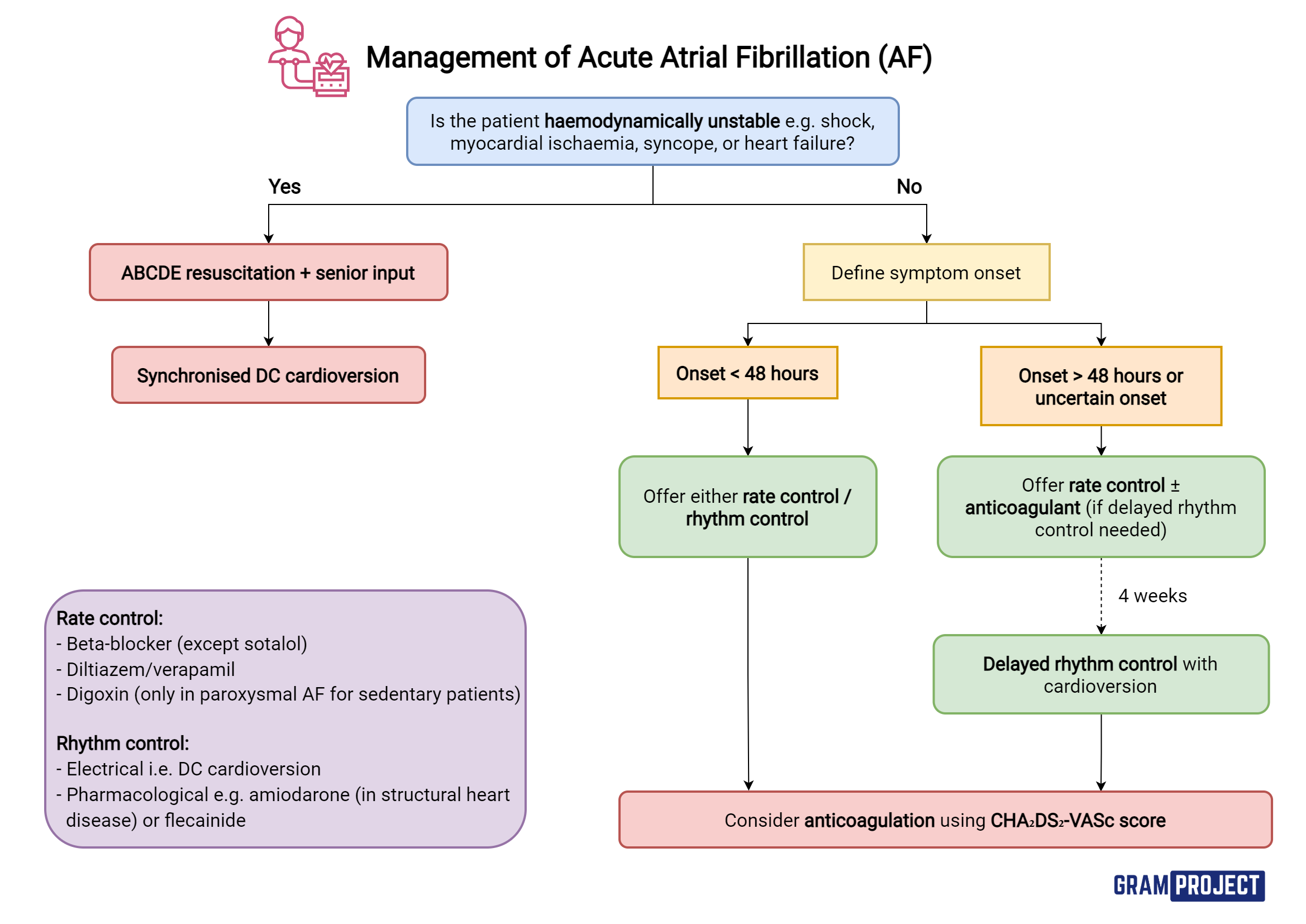
If haemodynamically unstable = emergency electrical cardioversion
Haemodynamically unstable = dangerous HR or low BP
Do not worry about anticoagulation before cardioversion yet
If urgent rate control required = beta blocker or verapamil - via IV
If not emergency - consider how long arrhythmia has been present
Rate control initially - because of risk of clot (blood pooling in heart) + causing stroke
For rhythm control – IV amiodarone or flecainide can be used in new onset AF
Amiodarone preferred in structural heart disease
Digoxin - non-paroxysmal AF ONLY if sedentary (little/no exercise)
In AF > 48h – with plan for long-term rhythm control:
Delay cardioversion until maintained on therapeutic anticoagulation for min. 3 weeks
Offer rate control during this period as appropriate
What is the aim of rate and rhythm control?
Rate control aims to get the heart rate below 100 and extend the time during diastole for the ventricles to fill with blood.
Rhythm control aims to return the patient to normal sinus rhythm.
When should rate control NOT be offered?
All patients with AF should have rate control as first-line, except with:
A reversible cause for their AF
New onset atrial fibrillation (within the last 48 hours)
Heart failure caused by atrial fibrillation
Symptoms despite being effectively rate controlled
Options for rate control?
Beta blocker first-line (e.g., atenolol or bisoprolol) - NOT sotalol
Calcium-channel blocker (e.g., diltiazem or verapamil) (not preferable in heart failure)
Digoxin (in non-paroxysmal AF ONLY if sedentary (little/no exercise)
Monotherapy with one.
if not controlling symptoms and if continuing symptoms are due to poor ventricular rate control:
Consider combo therapy with any TWO of:
BB, diltiazem or digoxin
Do NOT offer amiodarone for long-term rate control
When should rhythm control be offered?
To patients with:
A reversible cause for their AF
New onset atrial fibrillation (within the last 48 hours)
Heart failure caused by atrial fibrillation
Symptoms despite being effectively rate controlled
Options for rhythm control?
Cardioversion (immediate or delayed)
Long-term rhythm control using medications
What is immediate cardioversion?
Used if the atrial fibrillation is either:
Present for less than 48 hours
Causing life-threatening haemodynamic instability
Two options for immediate cardioversion:
Pharmacological cardioversion (flecainide or amiodarone)
Electrical cardioversion (shocks the heart back into sinus rhythm)
What is delayed cardioversion? Considerations?
Used if the atrial fibrillation has been present for more than 48 hours and they are stable. Electrical cardioversion is recommended.
Amiodarone therapy starting 4 weeks before (+ anticoagulation) + continuing for up to 12 months after
Patient should be anticoagulated for at least 3 weeks before delayed cardioversion.
During the 48 hours before cardioversion - may have developed a blood clot in the atria, and reverting them to sinus rhythm carries a high risk of mobilising that clot, causing a stroke. They are rate controlled whilst waiting for cardioversion.
How is long-term rhythm control achieved?
Beta blockers first-line
Dronedarone second-line for maintenance of sinus rhythm – after successful cardioversion in those with paroxysmal or persistent AF
Amiodarone is useful in patients with heart failure or left ventricular dysfunction
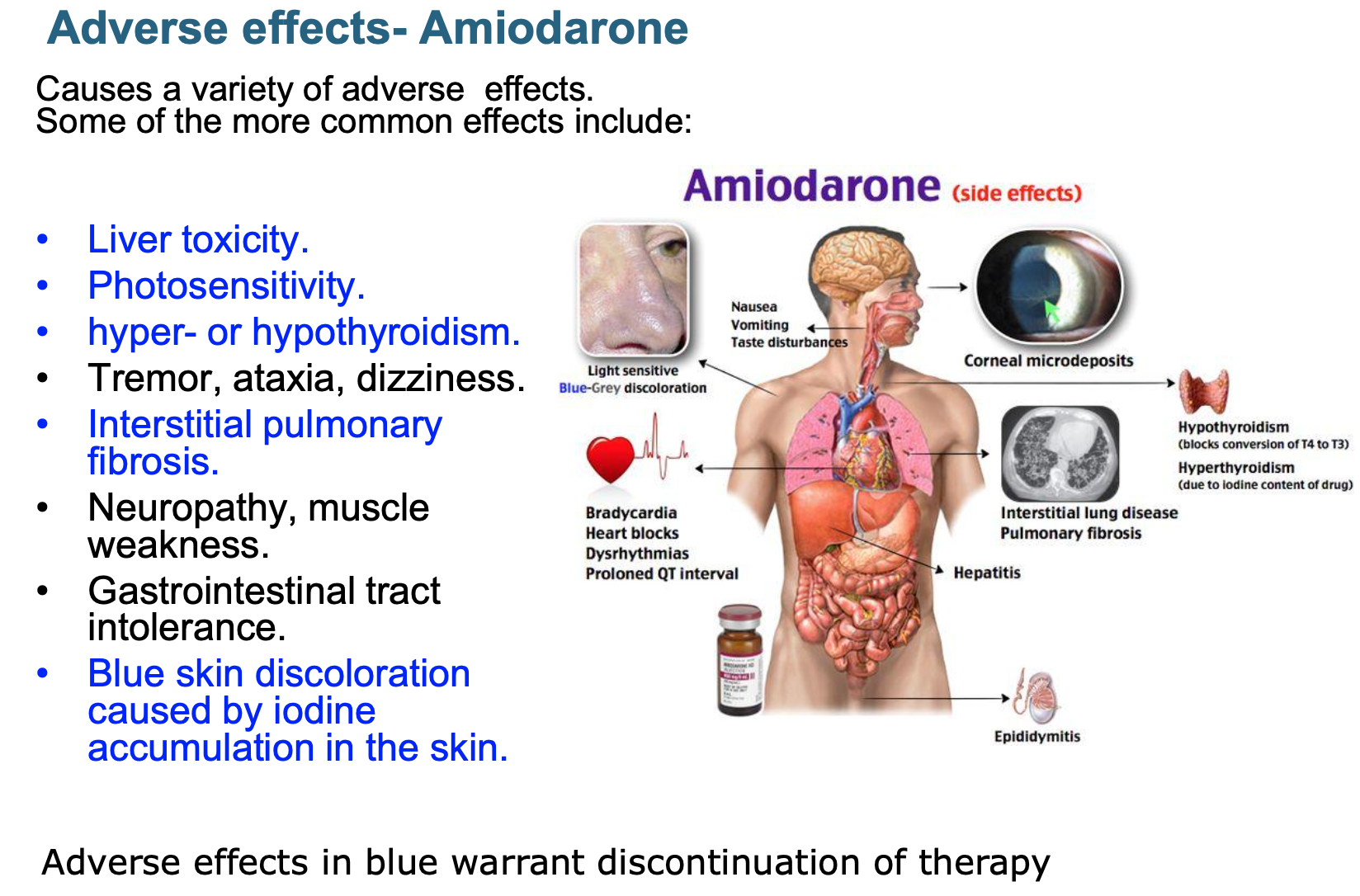
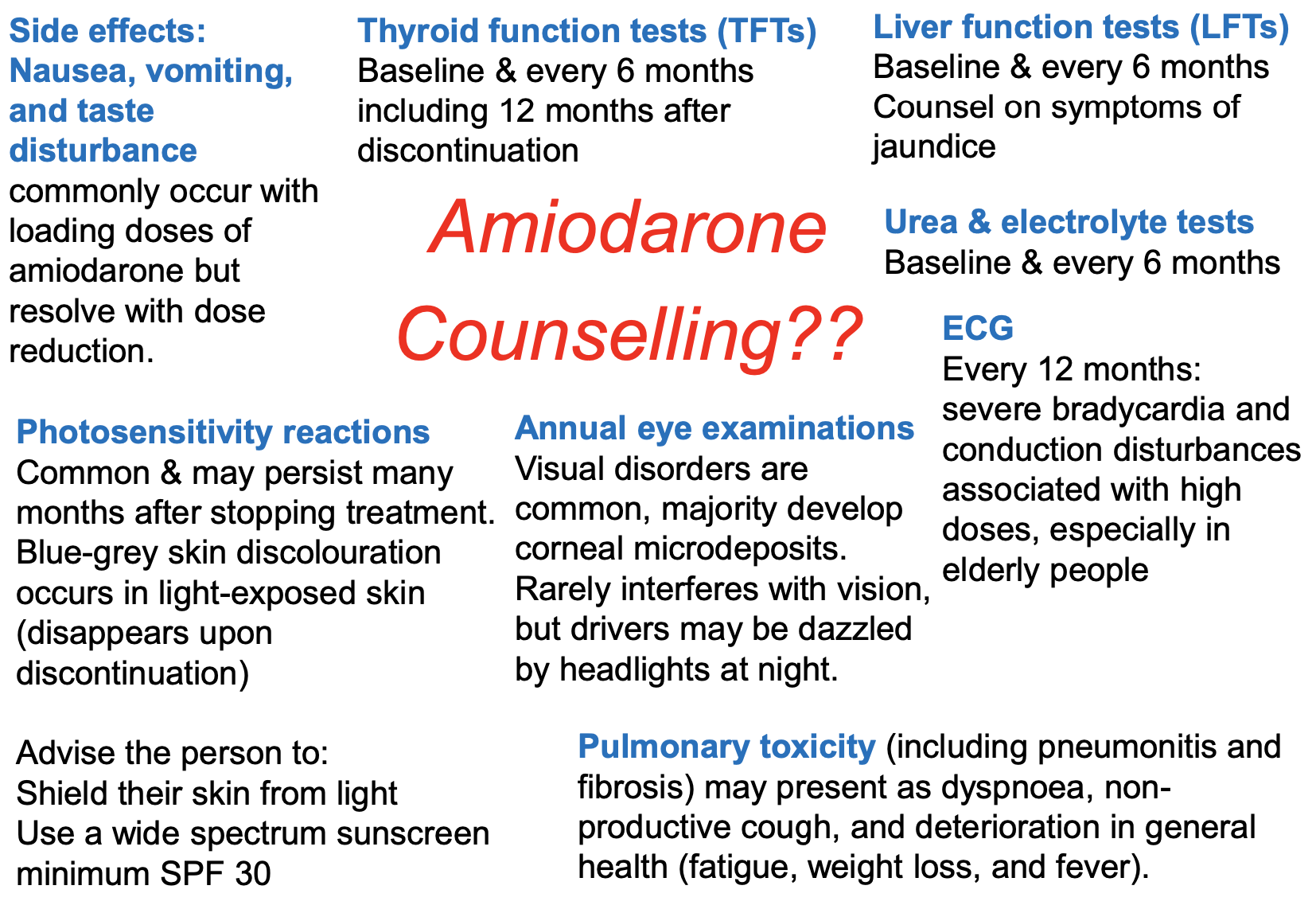
Features of amiodarone? Adverse effects?
Rate controlling + antiarrhythmic activity but S/E mean it is not used much for rate control
Contains iodine + structurally related to thyroxine (so effect on thyroid gland)
Requires extensive loading
Prolonged half-life of several weeks
Distributes extensively in adipose tissue
Full clinical effects may not be achieved until 6 weeks after initiation
Due to long half-life
Counselling for amiodarone?
How can paroxysmal atrial fibrillation be managed?
“pill-in-the-pocket” approach:
take a pill to terminate AF only when feel the symptoms starting.
Patient must:
have infrequent episodes without structural heart disease.
be able to identify the signs of AF and when to take treatment.
Flecainide - usual choice:
risk of flecainide converting the atrial fibrillation into atrial flutter, with 1:1 AV conduction to the ventricles, causing a very fast ventricular rate.
Patients with paroxysmal atrial fibrillation should still be anticoagulated based on their CHA2DS2-VASc score, similar to permanent atrial fibrillation.
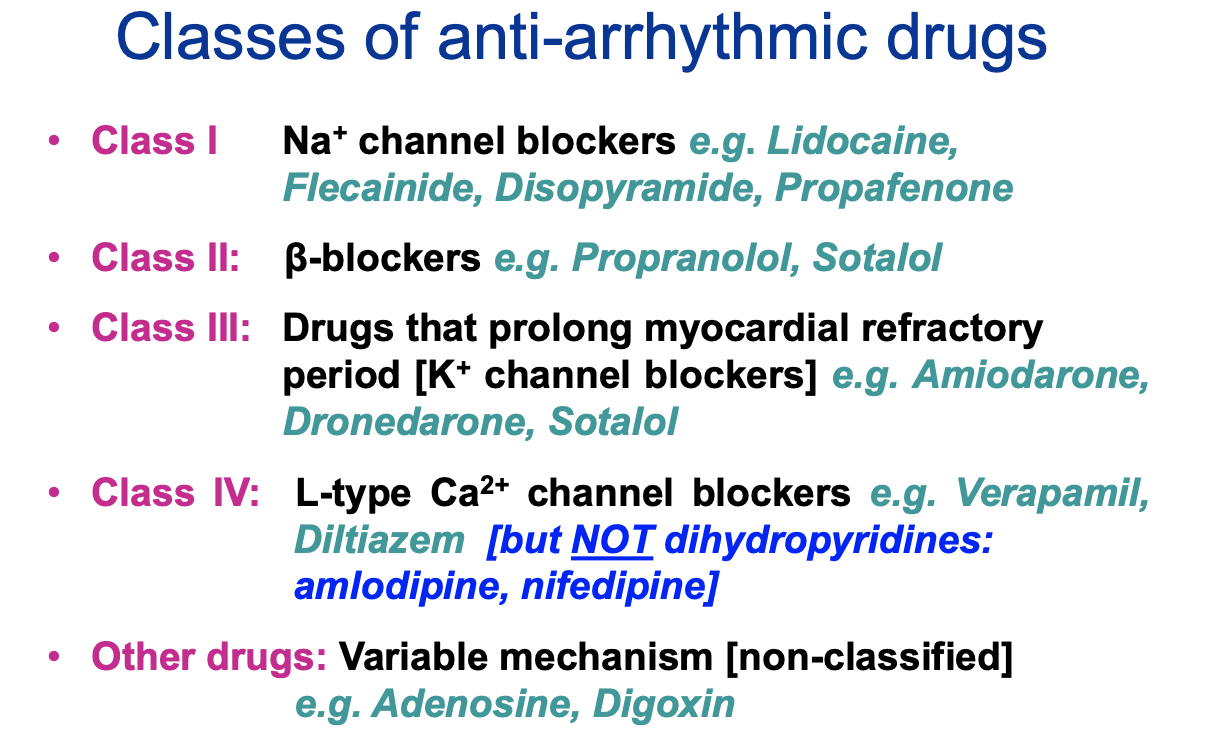
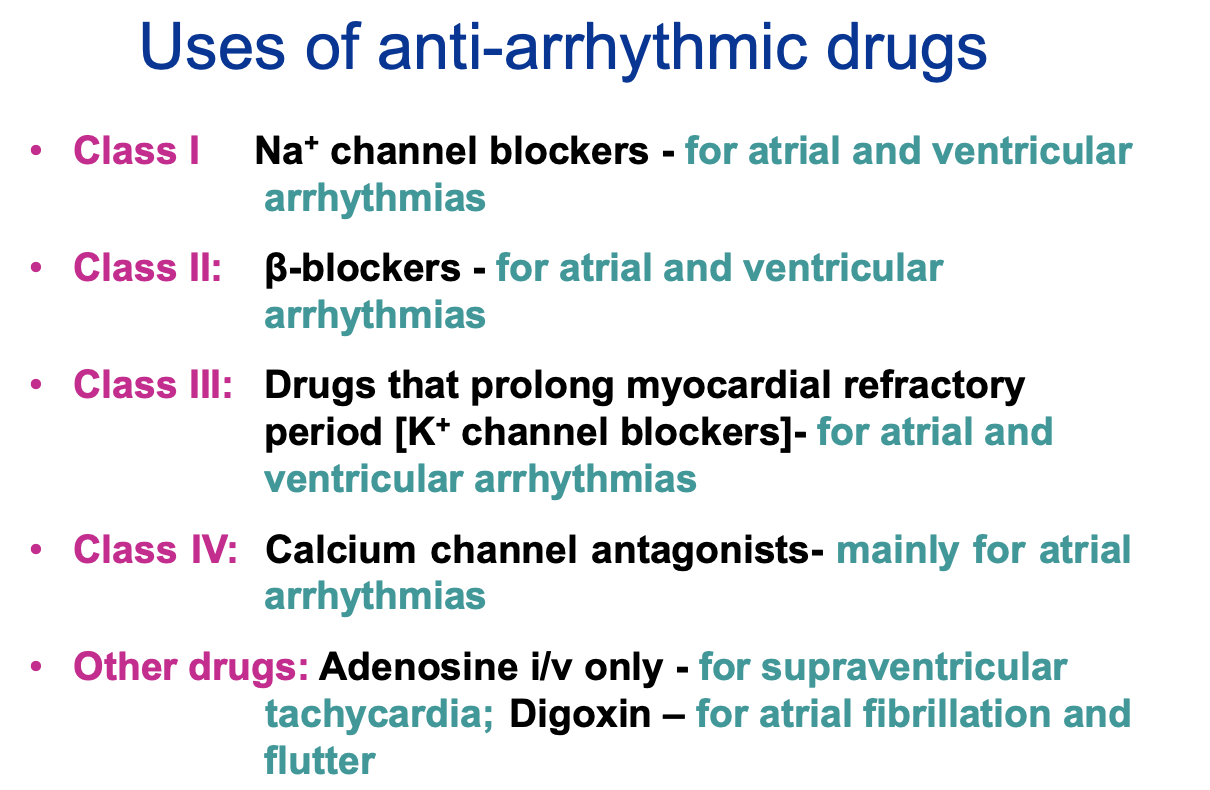
What are the classes of anti-arrhythmic drugs?
How can stroke risk in AF be assessed?
CHA2DS2-VASc is a tool for assessing whether a patient with atrial fibrillation should start anticoagulation.
Higher the score = higher the risk of developing a stroke or TIA.
CHA2DS2-VASc is a mnemonic for the factors that score a point:
C – Congestive heart failure
H – Hypertension
A2 – Age above 75 (scores 2)
D – Diabetes
S2 – Stroke or TIA previously (scores 2)
V – Vascular disease
A – Age 65 – 74
S – Sex (female)

1st line anticoagulant = DOAC
How can bleeding risk in AF be assessed?
ORBIT bleeding risk score if considering starting anticoagulation in AF.
O – Older age (age 75 or above)
R – Renal impairment (GFR less than 60)
B – Bleeding previously (history of gastrointestinal or intracranial bleeding)
I – Iron (low haemoglobin or haematocrit)
T – Taking antiplatelet medication
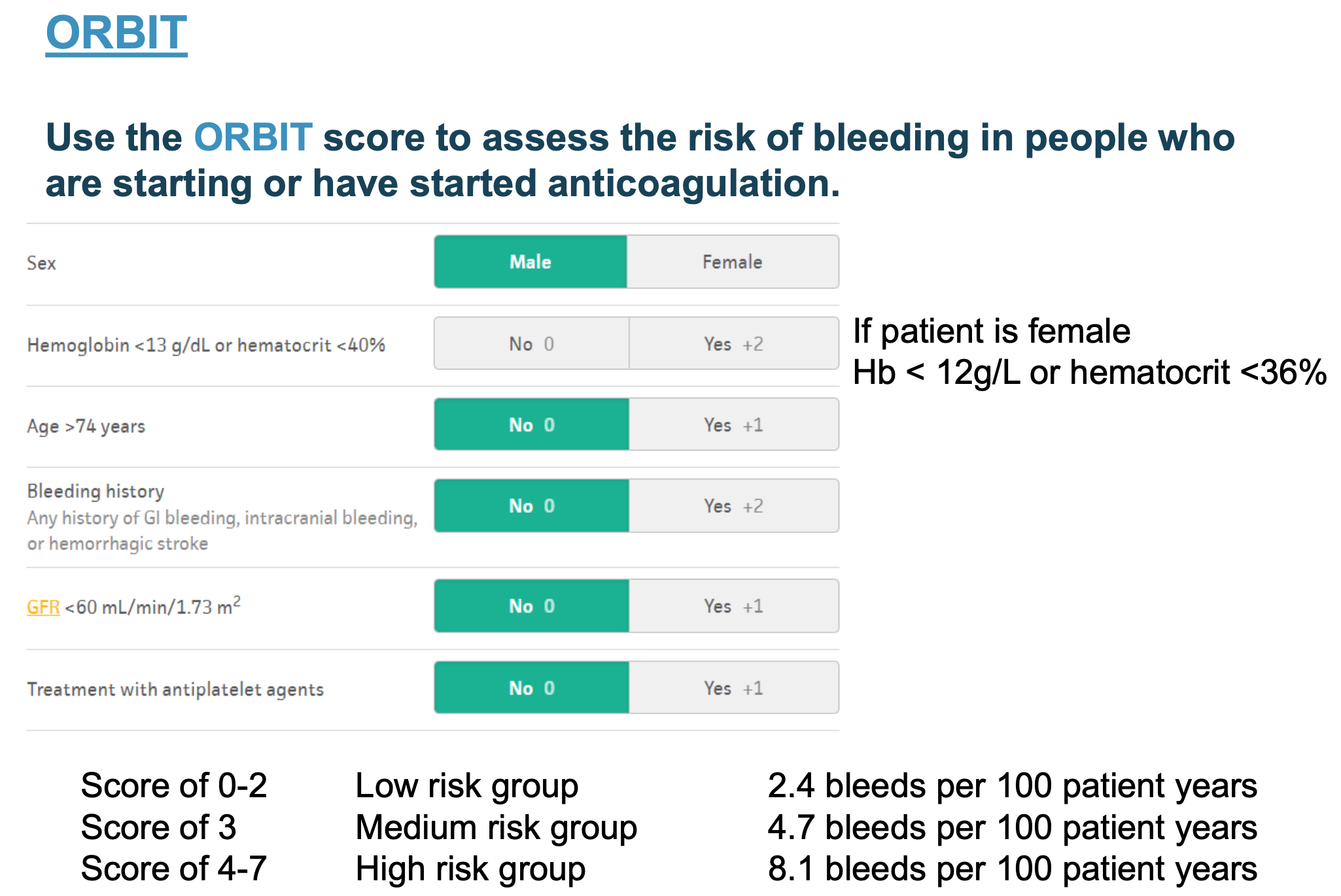
How is anticoagulation treatment recommended?
Direct-acting oral anticoagulants (DOACs) first-line
Warfarin second-line, if DOACs are contraindicated
Anticoagulation treatment reduces coagulation (thrombus formation) by interfering with the clotting cascade.
Features are DOACs?
Oral anticoagulants that do not require INR monitoring, unlike warfarin.
are suitable for most patients, incl. patients with cancer.
have a 6-14 hour half-life.
Direct factor Xa inhibitors: apixaban, edoxaban and rivaroxaban
Direct thrombin inhibitor: dabigatran
Taken twice daily: apixaban and dabigatran
Taken once daily: edoxaban and rivaroxaban
Reversal agent for DOACs?
Andexanet alfa (apixaban and rivaroxaban) - aka Ondexxya
Idarucizumab (a monoclonal antibody against dabigatran)
MoA of warfarin?
Warfarin is a vitamin K antagonist.
Vitamin K is essential for the functioning of several clotting factors.
Warfarin blocks vitamin K.
Prolonging prothrombin time (time it takes for blood to clot).
Features of warfarin?
INR - used to assess how anticoagulated the patient is by warfarin.
requires close monitoring of INR and frequent dose adjustments to keep the INR in range.
given once daily - so the INR is available before deciding dose.
target INR for AF is 2 – 3 (2.5).
How is warfarin metabolised? What affects it?
metabolism of warfarin involves cytochrome P450 system in the liver.
INR will be affected by other drugs that influence the activity of the P450 system
when starting new medications - INR needs close monitoring, and the warfarin dose needs to be adjusted accordingly.
INR is affected by many foods, esp. those containing vitamin K:
e.g., leafy green vegetables, and those that affect the P450 system, such as cranberry juice and alcohol.
must monitor INR more closely when patient changes their diet.
What does INR mean?
INR - calculates the patient’s prothrombin time compared with the prothrombin time of an average healthy adult.
INR of 1 indicates a normal prothrombin time.
INR of 2 means the patient has a prothrombin time twice that of an average healthy adult (it takes them twice as long to form a blood clot).
What is time in therapeutic range (TTR)?
Refers to the percentage of time that the INR is in the target range.
When the INR is too low - increased risk of a stroke.
When the INR is too high - increased risk of bleeding.
How can effects of warfarin be reversed?
Vitamin K - in the event of a very high INR or significant bleeding.
Warfarin has a half-life of 1-3 days.
water soluble vitamin K (menadiol)
What is atherosclerosis?
A condition caused by plaque buildup (fat, cholesterol, and other substances) in the artery walls.
Over time - plaque causes arteries to harden and narrow, reducing blood flow.
May rupture, leading to blood clots.
How does atherosclerosis occur?
caused by chronic inflammation and activation of the immune system (macrophages) in the artery wall.
causes deposition of lipids (mostly LDL) in the artery wall
followed by fatty deposits in artery walls.
Modifiable and non-modifiable risk factors for CVD?
Modifiable risk factors:
Raised cholesterol
Smoking
Alcohol consumption
Poor diet
Lack of exercise
Obesity
Poor sleep
Stress
BP
Type II diabetes
Non-modifiable risk factors:
Older age
Family history
Male
Ethnic origin
What are direct CV markers?
High total cholesterol
High LDL cholesterol (bad cholesterol)
Low HDL cholesterol (good cholesterol)
High triglycerides
Other = C-reactive protein (CRP)

What is the difference between primary and secondary prevention of CVD?
Primary prevention for patients that have never had a diagnosis of CVD.
But are at risk of a first cardiovascular event.
If lifestyle change alone is ineffective or inappropriate in this group of people - atorvastatin 20 mg daily (high-intensity statin)
Secondary prevention to reduce risk of recurrent CV event e.g., angina, myocardial infarction, TIA, stroke or peripheral arterial disease.
Offer atorvastatin 80 mg daily, regardless of the person's cholesterol level
How to optimise modifiable risk factors?
Address diet, exercise and obesity
Stop smoking
Reducing alcohol consumption
Optimise treatment of co-morbidities (such as diabetes)
How should diet and exercise be modified?
Dietary changes:
Total fat is less than 30% of total calories (primarily monounsaturated and polyunsaturated fats)
Saturated fat is less than 7% of total calories
Reduced sugar intake
Wholegrain options
At least 5 a day of fruit and vegetables
At least 2 a week of fish (one being oily)
At least 4 a week of legumes, seeds and nuts
Exercise (limited by co-morbidities):
Aerobic activity for a total of at least 150 minutes at moderate intensity or 75 minutes at vigorous intensity per week
Strength training activities at least 2 days a week
What is QRISK score?
QRISK score estimates the percentage risk that a patient will have a stroke or myocardial infarction in the next 10 years.
When the result is above 10%, they should be offered a statin, initially atorvastatin 20mg at night.
Which patients are offered atorvastatin 20mg as primary prevention?
QRISK of 10% or more (incl. people with type 2 diabetes)
Chronic kidney disease (eGFR less than 60 ml/min/1.73 m2)
Type 1 diabetes for more than 10 years or are over 40 years
Familial hypercholesterolemia.
Aged 85 and older.
What is the lipid target in primary and secondary prevention?
Primary - lipid target is a greater than 40% reduction in non-high-density lipoprotein (non-HDL) cholesterol.
If target is not met and atorvastatin is used - increase atorvastatin dose every 2–3 months up to a max. daily dose of 80 mg.
if still out of target after 2-3 months - consider adding ezetimibe.
Secondary - lipid target is LDL cholesterol levels of 2.0 mmol/L or less or non-HDL cholesterol levels of 2.6 mmol/L or less.
MoA of statins?
Statins reduce the synthesis of cholesterol in the liver by competitively inhibiting HMG CoA reductase (rate-limiting enzyme of cholesterol biosynthesis)
Reduction in intracellular cholesterol promotes increased low-density lipoprotein (LDL) receptor expression at the surface of the hepatocytes.
Resulting in increased uptake of LDL from the blood and decreased plasma concentrations of LDL- and other ApoB-containing lipoproteins.
Important side effects of statins?
Myopathy (causing muscle weakness and pain)
Rhabdomyolysis (muscle damage – check the creatine kinase in patients with muscle pain)
Type 2 diabetes (hyperglycaemia)
Haemorrhagic strokes (very rarely)
Important interactions/contraindications with statins?
Interaction - macrolide antibiotics - clarithromycin or erythromycin. Grapefruit.
CI - pregnancy and breastfeeding. Active liver disease or persistent high transaminases (3 times upper limit).
women of childbearing potential - require adequate contraception during treatment.
stop 3 months before attempting to conceive and during pregnancy.
MoA of ezetimibe?
Inhibits intestinal uptake of dietary and biliary cholesterol without affecting the absorption of fat-soluble nutrients.
By inhibiting cholesterol absorption, ezetimibe reduces the amount of cholesterol delivered to the liver.
Reduced cholesterol delivery promotes increased low-density lipoprotein (LDL) receptor expression.
Leading to increased clearance of LDL cholesterol from the blood.
I.e., inhibits absorption of cholesterol in the intestine
When is ezetimibe used?
As an alternative when statins are not tolerated or in combination with a statin when statins alone are inadequate.
Ezetimibe may be combined with bempedoic acid (drug that reduces cholesterol production in the liver) - when lipid target not met.
What are other lipid treatments?

What treatments are used for secondary prevention of CVD?
“4 As” mnemonic:
A – Antiplatelet medications (e.g., aspirin, clopidogrel and ticagrelor)
A – Atorvastatin 80mg
A – Atenolol (or an alternative beta blocker – commonly bisoprolol) titrated to the maximum tolerated dose
A – ACE inhibitor (commonly ramipril) titrated to the maximum tolerated dose
After a myocardial infarction, patients are offered dual antiplatelet treatment initially, with:
Aspirin 75mg daily (continued indefinitely)
Clopidogrel or ticagrelor (generally for 12 months before stopping)
How is thrombus (arterial and venous) prophylaxis achieved in CVD?
Arterial thrombi – interrupt blood flow – causing ischaemia or death of tissue beyond thrombi
Usually associated with atherosclerosis
Aspirin – reduce platelet rich arterial thrombi
Inhibits COX-1 – reducing synthesis of thromboxane TXA2 (stimulant of platelet aggregation made by platelets)
Reduces synthesis of prostacyclin PGI2 (inhibitor of aggregation made by vascular endothelium)
Increases + decreases platelet aggregation – endothelium responsible for making PGI2 can make more PGI2 after COX-1 has been inhibited (reduced first then increased)
Platelets do not have nucleus so cannot produce more TXA2 – when TXA2 inhibited, new platelets have to made in order for TXA2 to be produced
Venous thrombi – fewer platelets + large component of fibrin
Treated with warfarin + heparin
What is heart failure?
Refers to the clinical features of impaired heart function, specifically the function of the left ventricle to pump blood out of the heart and around the body.
Causes of heart failure?
HF caused by left ventricular systolic dysfunction
LV (pumps blood around body) weakens - insufficient delivery of blood to organs.
HF with preserved ejection fraction (HFPEF)
LV becomes stiff – difficult for heart chamber to fill with blood
Ejection fraction (EF) = normally between 50-70%
= amount of blood pushed out of LV every time heart beats
Never 100% - always blood left in heart
Reduced EF - 40% or less - LV loses ability to contract normally
Preserved ejection fraction = LV loses ability to relax normally during diastole (stiff)
EF greater than 50% - but has clinical features of HF
Key symptoms/signs of chronic HF?
Symptoms
Breathlessness - by exertion, at rest, when lying flat (orthopnoea), nocturnal cough, or paroxysmal nocturnal dyspnoea (patient wakes suddenly, gasping for air)
Ankle swelling/fluid retention
Fatigue
Lightheadedness
Signs:
Elevated jugular venous pressure
Basal crepitations
Peripheral oedema
What assessments are done to help diagnose HF?
Clinical assessment (history and examination)
N-terminal pro-B-type natriuretic peptide (NT‑proBNP) blood test
ECG
Echocardiogram
Other investigations include:
Bloods for anaemia, renal function, thyroid function, liver function, lipids and diabetes
Urinalysis
Chest x-ray and peak flow/spirometry to exclude lung pathology
What is the New York Heart Association Classification (NYHA)?
Used to grade the severity of symptoms related to heart failure.
Class I: No limitation on activity
Class II: Comfortable at rest but symptomatic with ordinary activities
Class III: Comfortable at rest but symptomatic with any activity
Class IV: Symptomatic at rest
How can NT-proBNP result be used to assess urgency for referral?
From 400 – 2000 ng/litre should be seen and have an echocardiogram within 6 weeks
Above 2000 ng/litre should be seen and have an echocardiogram within 2 weeks
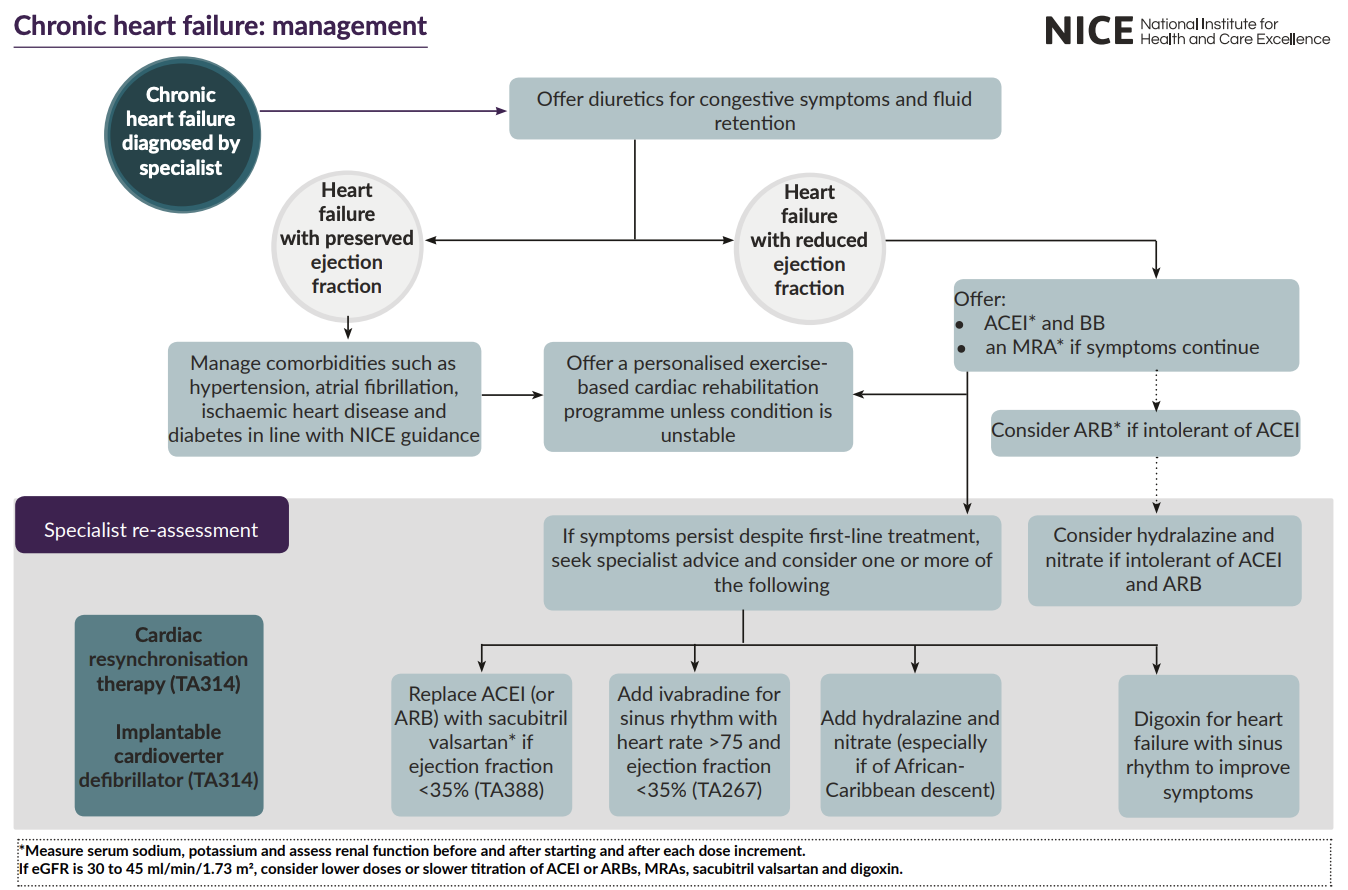
Pharmacological treatment for chronic HF?
Loop diuretic e.g., furosemide or bumetanide - offer first to manage oedema.
May need to reduce dose to maintain BP after addition of e.g., BB.
ACEi - start low + titrate up in short intervals e.g., every 2 weeks.
Do NOT initiate if suspected valve disease.
May be preferred if have diabetes or has signs of fluid overload (BB may make the symptoms of HF worse).
If intolerable e.g., cough - offer ARB.
Take first dose at night to avoid dizziness from low BP.
BB - start low + go slow.
Switch stable pts already taking BB for comorbidity to BB licenced for HF e.g., bisoprolol, carvedilol.
S/E’s: fatigue, cold hands + feet, dizziness.
MRA - mineralocorticoid receptor antagonist (MRA) if remain symptomatic.
MoA: lowers BP but lowers circulating levels of aldosterone (which is raised in HF) - reduces stiffening of heart
E.g., spironolactone or eplerenone.
Epelerenone if ejection fraction < 35%.
Can also add an SGLT-2 inhibitor (empagliflozin or dapagliflozin) - if symptoms persist despite 1st line treatment.
N.b., extra but advice on flu and pneumonia vaccine.
Considerations/counselling for digoxin?

Considerations/counselling for ivabradine?

What medications should be reviewed in HF?
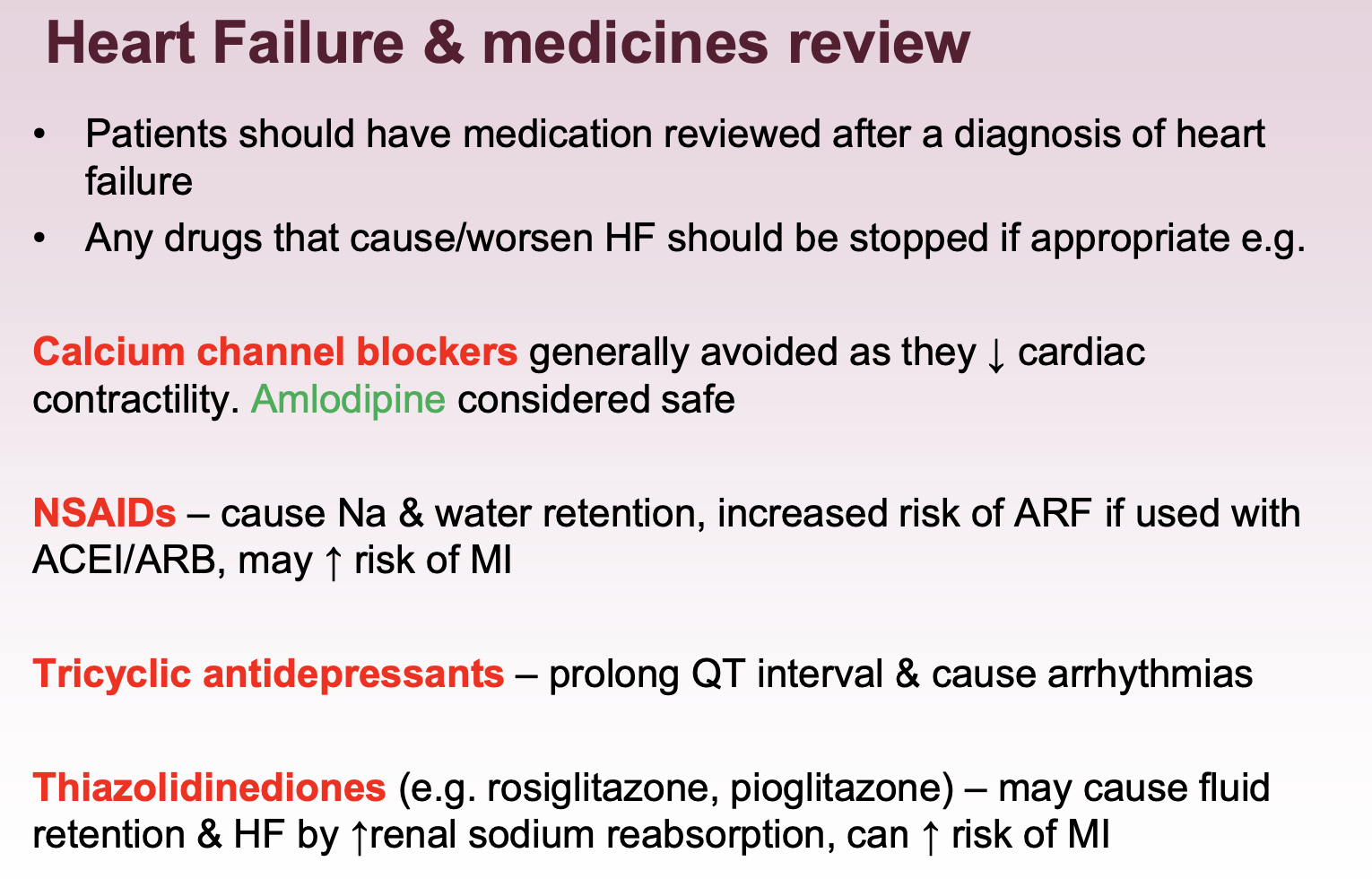
N.b., self management advice for HF - encourage daily weight checks - report weight gain > 1.5-2 kg, increase in SOB, and fluid accumulation.
Why are opiates, nitrates, inotropes/vasopressors, sodium nitroprusside (vasodilator) not offered for HF?

What is angina? Angina symptoms?
Angina is chest pain (or constricting discomfort) caused by an insufficient blood supply to the myocardium.
is the main symptom of myocardial ischaemia.
stable or unstable.
usually caused by coronary heart disease (CHD) i.e., atherosclerosis.
when high demand e.g., exercise - insufficient supply of blood to meet demand = angina symptoms.
Symptoms: constricting chest pain, with or without radiation to the jaw or arms.
How to classify symptoms of angina based on their typicality?
Typical angina presents with all three of the following features:
Precipitated by physical exertion.
Constricting discomfort in the front of the chest, in the neck, shoulders, jaw, or arms.
Relieved by rest or glyceryl trinitrate (GTN) within about 5 minutes.
Atypical angina presents with two of the above features.
In addition, atypical symptoms include gastrointestinal discomfort, and/or breathlessness, and/or nausea.