Biochemistry Lecture 2
1/53
Earn XP
Description and Tags
Learning goals: How water participates in biochemical reactions, weak interactions, and how water molecules influence structure, self assembly, and properties of all cellular components
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
why is water the solvent of choice in biological systems?
able to absorb large amounts of heat (and remain at the same temp.)
helps regulate intracellular pH (bc has a pH = 7)
design drugs so they’re water-soluble
used for transport — delivers nutrients and removes waste/toxins from cells
ex: sweating cools us down and rids toxins
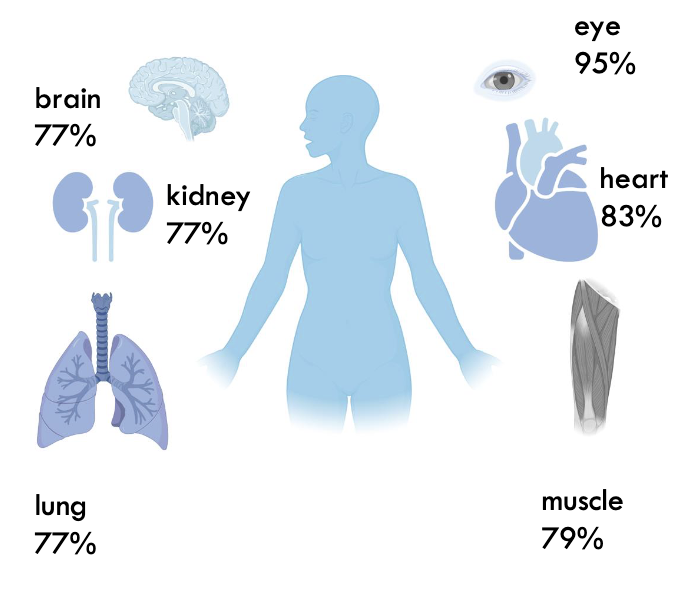
what percentage are we made of water?
~65%
what is “primordial soup”?
The first living organism existed in solution - had water and all necessary elements that assist in biological systems
What experiment brought about this theory?
1957 (actually 1952) - took methane gas, water, and some proponents of amino acids and heated it up - tried to replicate the heating of an organism itself - spontaneously formed amino acids - components used were not necessary for the origin of life but was a good guess
how do building blocks interact with water?
most building blocks are soluble in water
lipid bilayer (associate with each other rather than water)
3D shape of proteins
what is Brownian motion?
at any given time, water molecules are bouncing into each other, forming bonds/interacting, breaking those bonds
random movement of gases and liquids powered by background thermal energy
inside the cell supplies the energy for many of the interactions required for a functional biochemical system
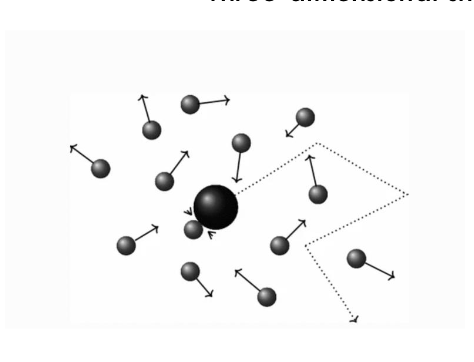
why is Brownian motion so important and what are the 2 components that allow for cellular interactions?
allows for cellular interactions
limited space
Brownian motion
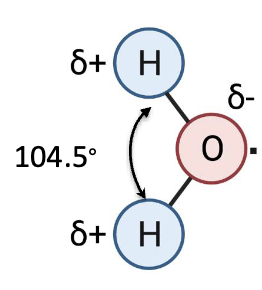
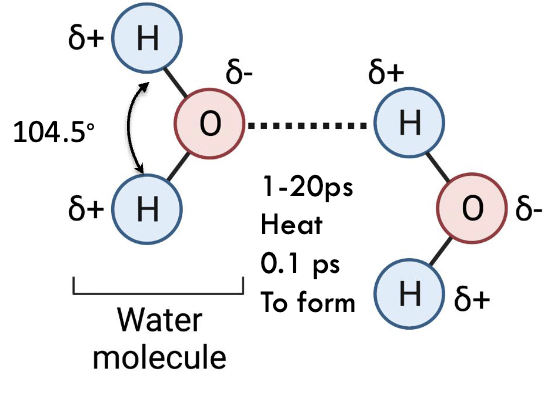
what shape does water have?
tetrahedral
what is the angle of the H-O-H bond?
104.5˚
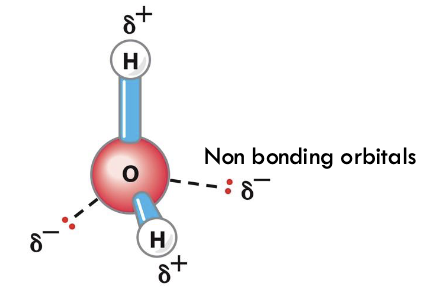
which is more electronegative, oxygen or hydrogen and what does this mean?
oxygen is more electronegative so H is considered electropositive - O has partial negative charge and H has partial positive - polar
what does the structure and its charges mean?
waters like to interact with each other through H-bonding
how long does contact between 2 water molecules last in warmer temps?
1-20 ps (picoseconds)
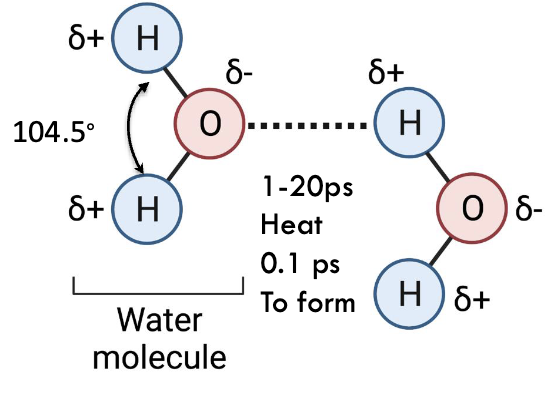
how long is the natural break H-bond between 2 water molecules?
0.1 ps
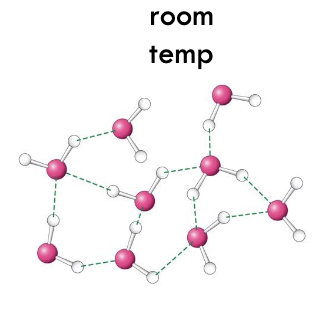
at room temp. at least how many water molecules interact and what are they not shaped like?
at least 3 molecules - average of 3.4 neighboring molecules - no lattice
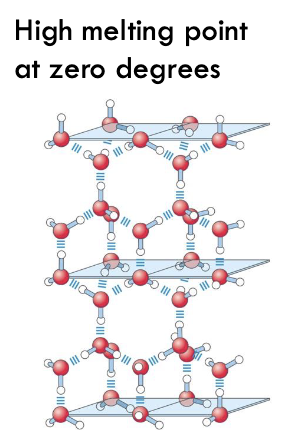
with ice, what structure and at least how many water molecules interact?
crystalline structure - at least 4 water molecules interacting
how does the bond dissociation energy of an H-bond compare to covalent bonds?
energy needed to break H-bond much less compared to covalent bonds
H-bond=8-23kJ/mol
Covalent bond (O-H)=470kJ/mol
Covalent bond (C-C)=348kJ/mol
Covalent bond (C-H)=418kJ/mol
What are some H-bond donors?
N-H and O-H (carbon bond between)
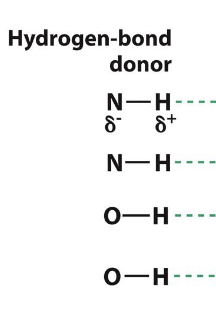
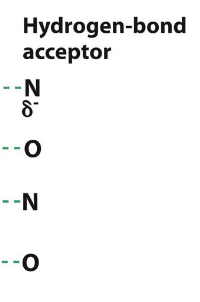
what are some H-bond acceptors?
N and O
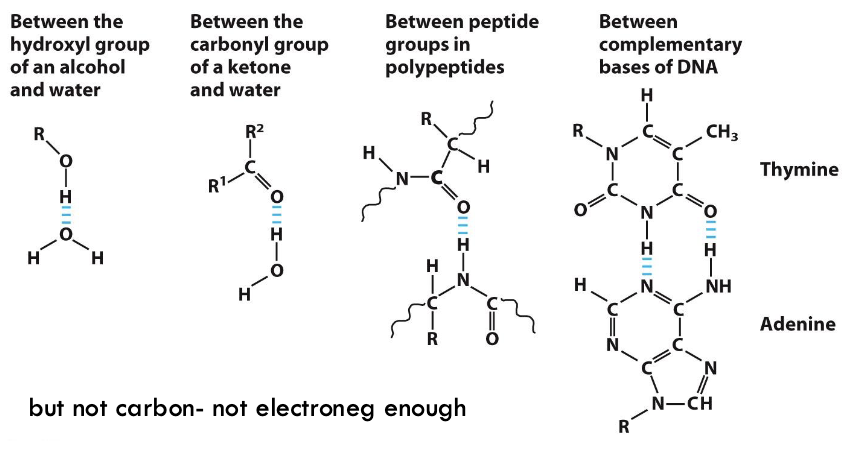
what are some examples of where we see H-bonding?
DNA helix between nucleotides and peptides
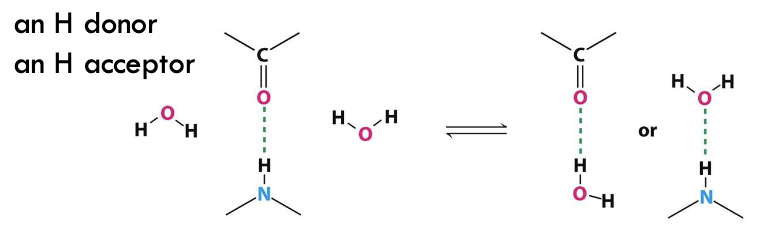
is water an H donor or H acceptor?
BOTH - universal
what is the distance of an H-bond?
2.4-3.5 angstroms
when is an H-bond strongest?
acceptor atom is in line with the covalent bond between the donor atom and H
maximizes electrostatic interaction
more constrained - less common
O, H, and O all on same plane
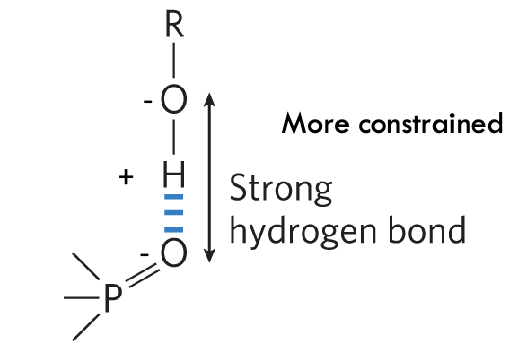
when is an H-bond weaker?
acceptor atom is at an angle with the covalent bond between the donor atom and H
less constrained
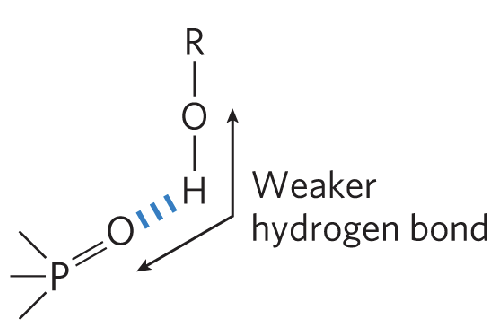
why are we more likely to see a weak H-bond rather than a strong H-bond?
macromolecules need to come together, form H-bonds, and break a part
important for:
replication (break DNA a part)
protein-protein interactions
what are the different classifications of compounds in relation to water?
hydrophilic, hydrophobic, and amphipathic
what are characteristics of a hydrophilic compound?
water-loving
dissolves easily in water
hydroxyl groups
anything that can form H-bonds with water
generally charged or polar compounds

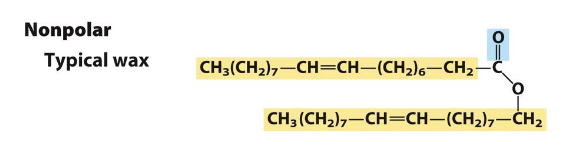
what are characteristics of a hydrophobic compound?
often have long hydrocarbon tails (don’t always dissolve or interact with water)
nonpolar molecules such as waxes
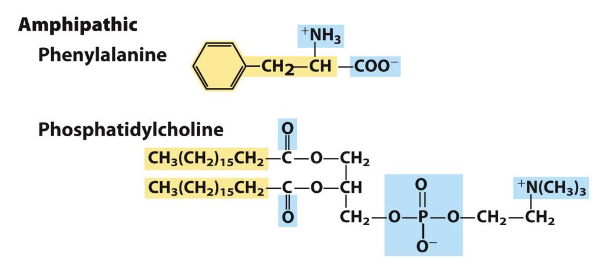
what are characteristics of an amphipathic compound?
contain polar and nonpolar regions such as lipids or some amino acids
what are electrostatic/ionic bonds and what is a common example?
interactions between oppositely charged ions - sodium chloride (salt)
how do we classify these interactions?
charge of the molecules
force needed to break interactions up in water
how does water interact with sodium chloride?
NaCl has lattice structure - water surrounds and interacts with each molecule
salt dissolve - individual ions now bind to water molecules rather than to each other
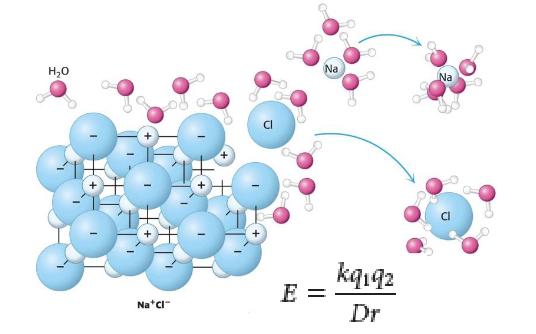
how do we measure electrostatic interactions and what are its variables?
Coulomb’s Law - the force that brings 2 compound together
q1 and q2: based on charge of 2 ions
k: energy needed to break up interactions (proportionality constant)
D: dielectric constant
when in vacuum = 1
when in presence of water, can be as high as 80 (water weakens electrostatic interactions)
r: distance
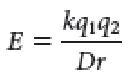
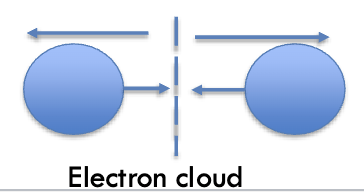
what are van der Waals interactions?
Relatively weak and reversible - involve attraction and repulsion - distance-dependent weak attractions and repulsions - as 2 nuclei draw closer, electron cloud begin to repel
What is van der Waals contact distance?
Transition from attraction to repulsion - point where electron cloud repels them away from each other
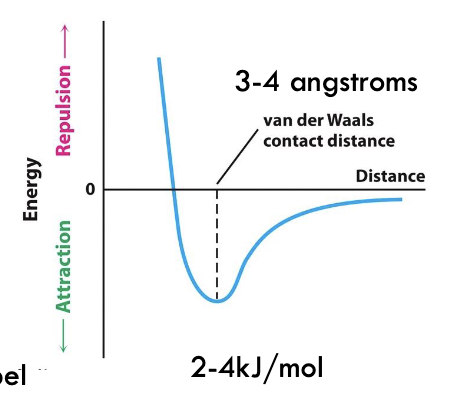
What is the amount of repulsion dependent on?
Type of ion
Size of electron cloud
What is entropy?
Degree of disorder or randomness
What happens when nonpolar molecules are in water?
Nonpolar molecules surrounded by water molecules
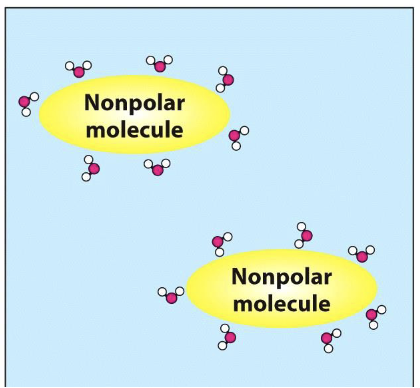
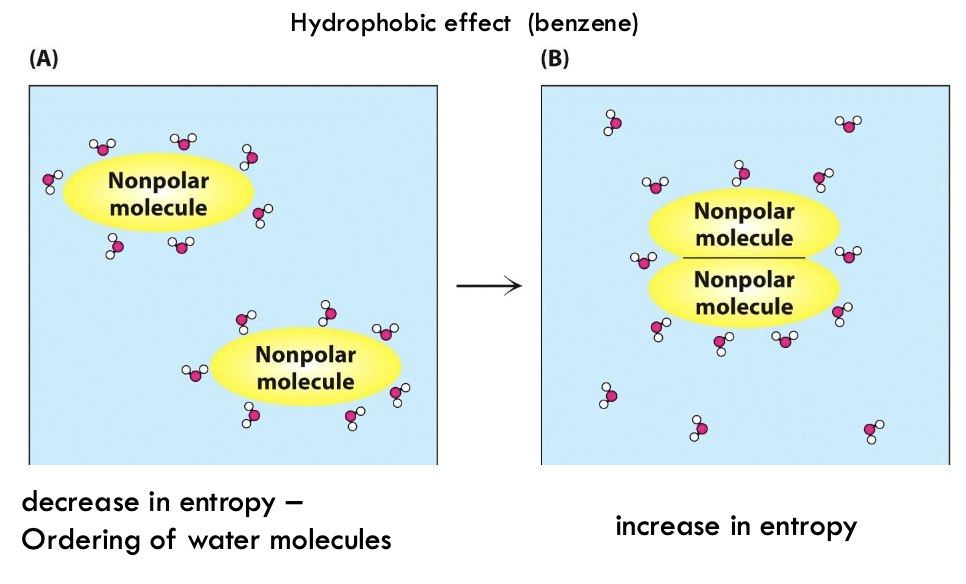
What happens to entropy when water molecules encase a nonpolar molecule?
Decrease in entropy - order of water molecules
What happens when 2 nonpolar molecules move together in water?
More random water interaction with each other - increase in entropy
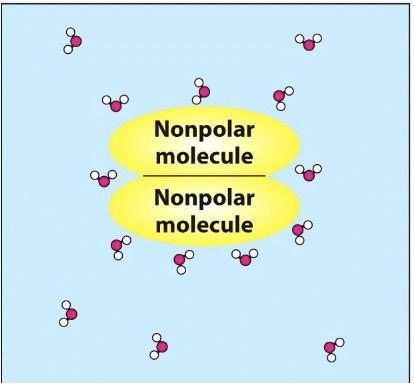
What is the hydrophobic effect and where does it occur?
Tendency of nonpolar molecules to aggregate in water, driven by the water molecules' preference to maximize their interactions with each other and minimize contact with nonpolar substances - oil and water, lipid bilayer
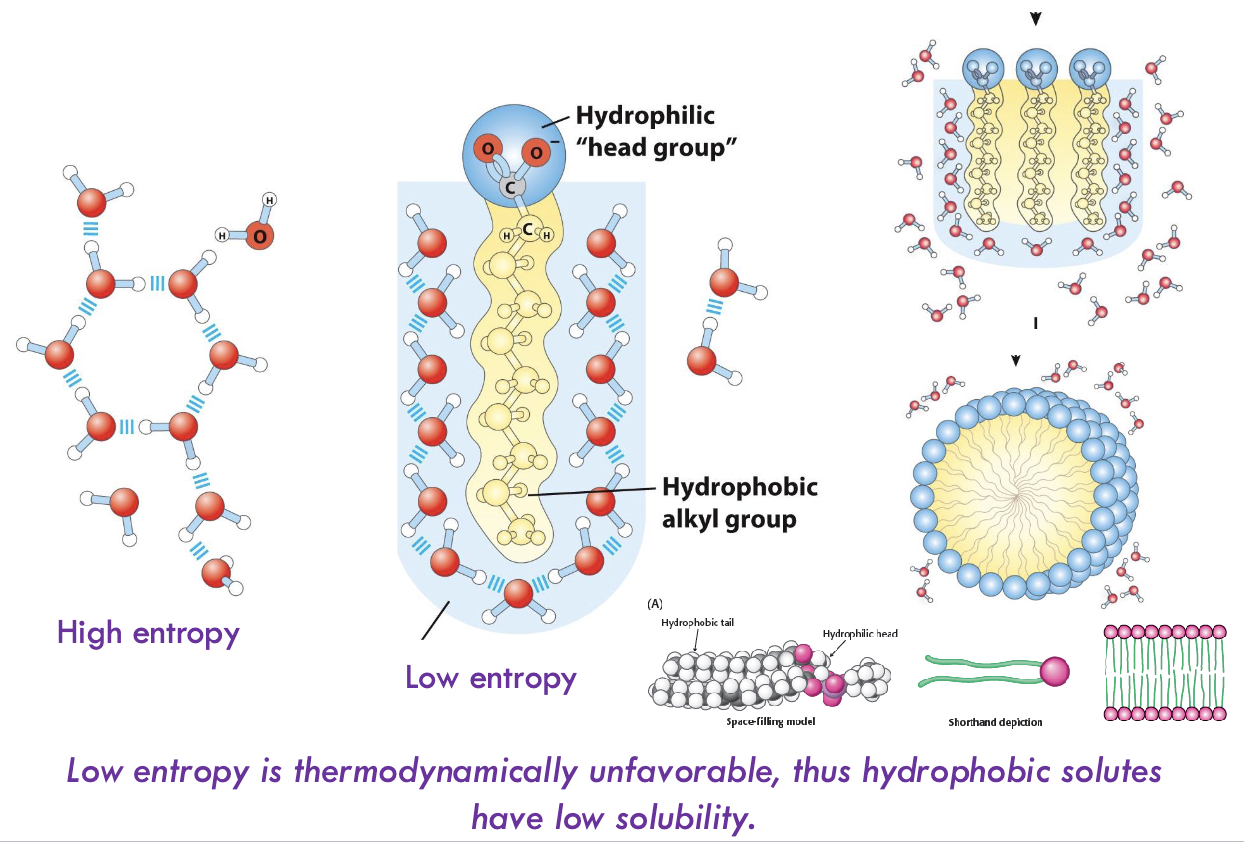
How does the hydrophobic effect occur with lipid bilayers?
Water molecules encase hydrophobic tails of lipids and drive the lipid tails together - from low entropy to high entropy - hydrophilic head groups interact with water molecules and with each other
What is another example of water molecules encasing?
Interactions between enzymes and substrates - enzyme and substrate surrounded by water molecules - have to have loss of water for 2 to interact
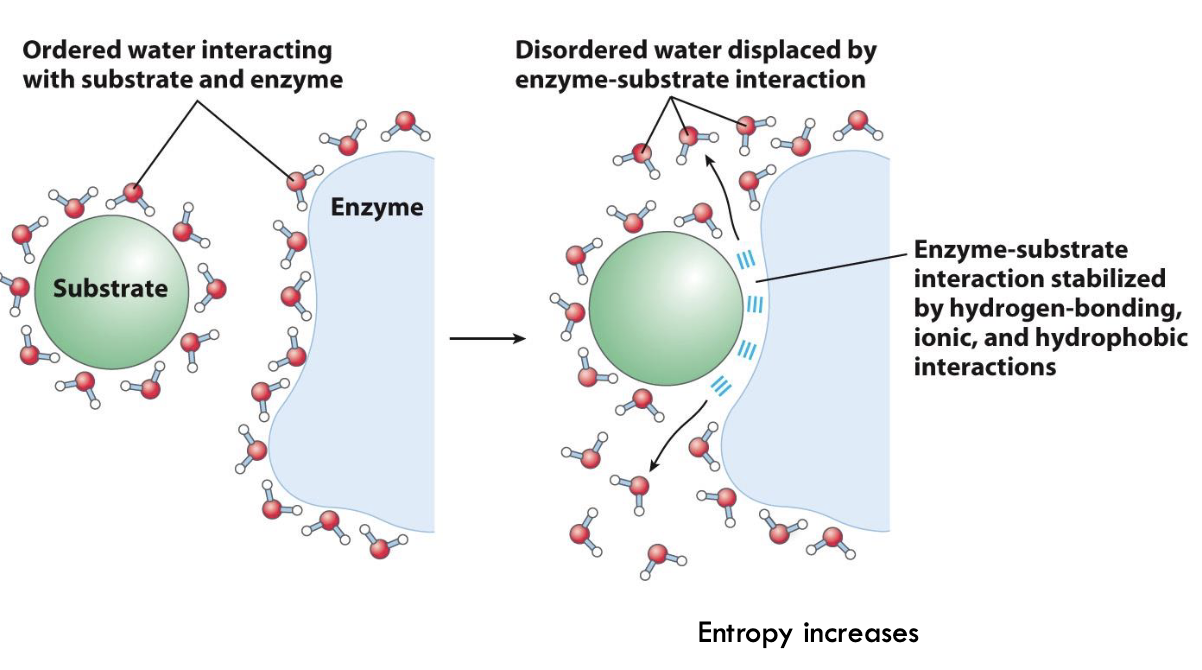
What helps enzymes-substrate interactions?
Brownian motion - move towards each other, loss of water molecules, able to bind and interact
How do macromolecules use weak interactions?
Use ionic, van der Waals to fold protein - H2O molecules are often found to be bound so tightly to biomolecules that they are part of the crystal structure
How does this apply to hemoglobin?
Residues on outside of hemoglobin interact with water molecules - drives folding and interactions of each polypeptide that make up hemoglobin
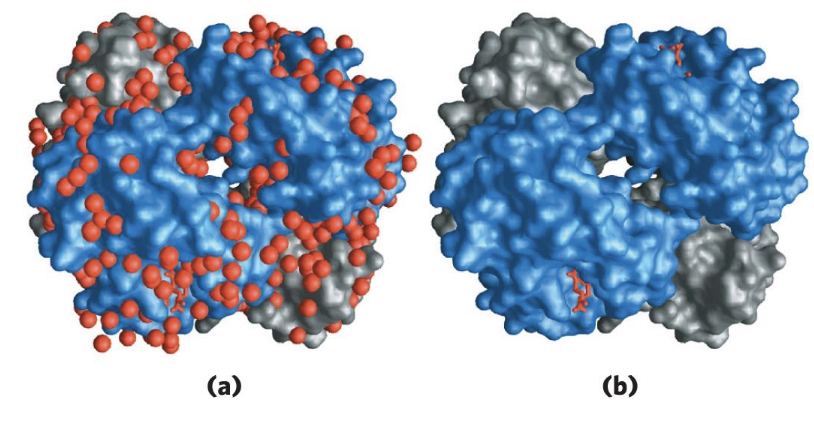
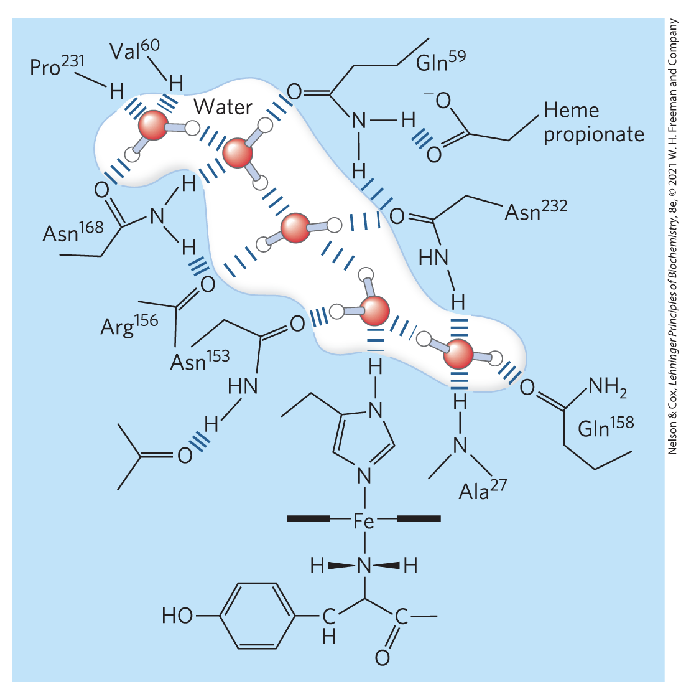
How is water essential to protein function and what is an example?
Proteins have water in active site, or water tunnel/chain being formed in active site
Cytochrome f
Has polywaters in its cavity - chain of 5 bound water molecules
Allows protons to travel into water molecules
May provide path for protons to move through the membrane
If no water, no transfer of protons
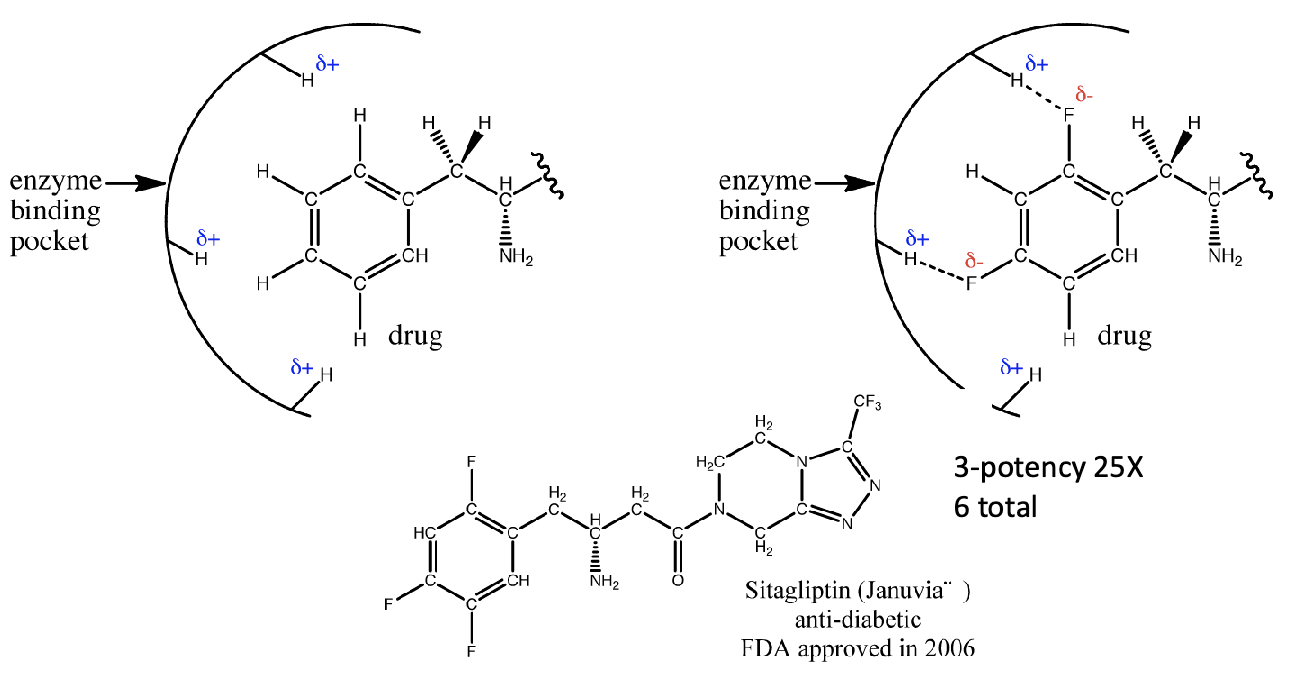
How is water essential in drug design?
Need to be able to dissolve drugs
What is an example of how drug design can increase efficency?
Januvia - Type 2 diabetes drug
Introduced fluorines molecules due to ability to H-bond
More fluorines —> more H-bonding
Increase strength of cavity - increase potency
From 0 to 3 to 6 fluorines - 25x potency
How can you describe the movement of water in and out of a cell?
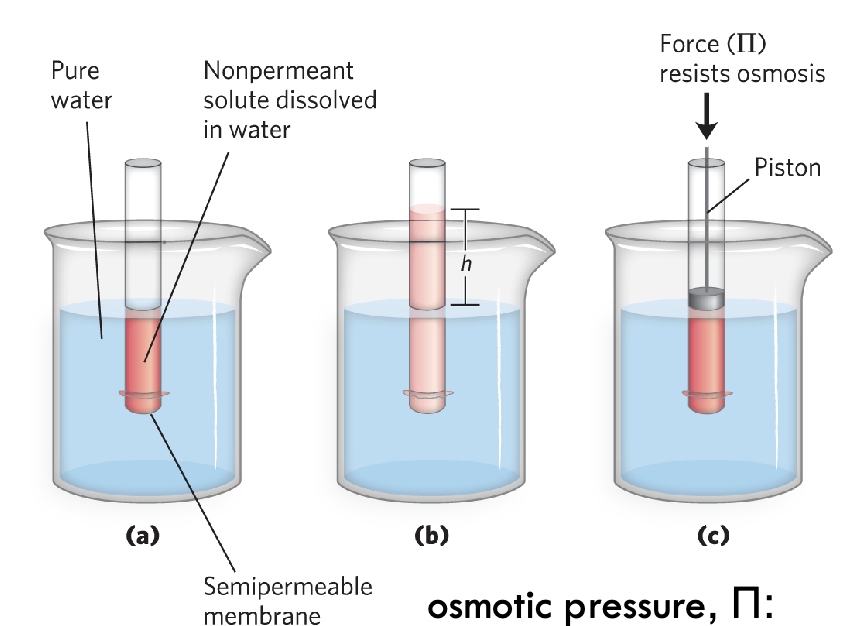
Osmotic pressure : force necessary to resist water movement
Representation of osmotic pressure
Tube with semipermeable membrane submerged in beaker of water
Membrane allows water in and out, but doesn’t allow solutes inside to leave tube
When water enters tube, displaces air and volume of h
If pressure added (osmotic force) - amount of force necessary to prevent water from traveling through
This prevention is cell membrane regulating transport of solution through lipid bilayer
What is an isotonic solution?
Extracellular and intracellular solutes are the same - transfer of water in equilibrium - cell stays same size
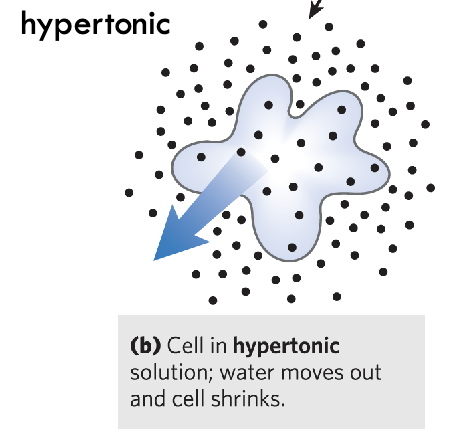
What is a hypertonic solution?
Water is moving out - cell is shrinking - imbalance of solution inside and outside of cell - ex. cell in salt water
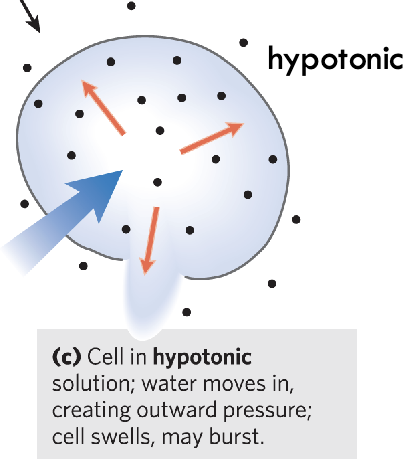
What is a hypotonic solution?
Solutes inside cell at higher conc. than solutes outside of cell - water rushing into cell and cell swells - cell could break
How is this prevented in natural biological systems?
Cell membrane/wall regulate solutes that transport across
Specific pumps (Na+ pumps - want to prevent salt from coming into cell)
Contractile vacuole