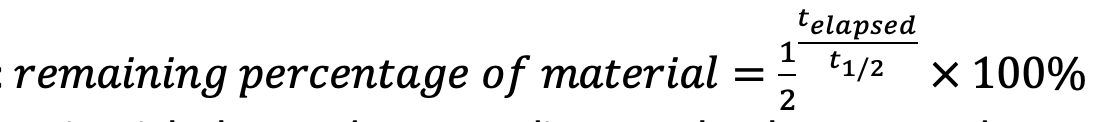option d - medicinal chemistry
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
Therapeutic index
The effectiveness and safety of drug
TI measures amount of drug needed to be consumed to produce overdose
If TI = 100, 100x the prescribed amount is needed to overdose
Effective dose (ED50)
minimum dose of drug to produce therapeutic effect
Toxic dose (TD50)
dose that causes toxicity in 50% of human patients
Lethal dose (LD50)
dose that causes death in 50% of lab animals
Therapeutic index in animal studies
the therapeutic index is the lethal dose divided by the minimum effective dose
LD50 / ED50
Therapeutic index in human studies
the therapeutic index is the toxic dose divided by the minimum effective dose
TD50 / ED50
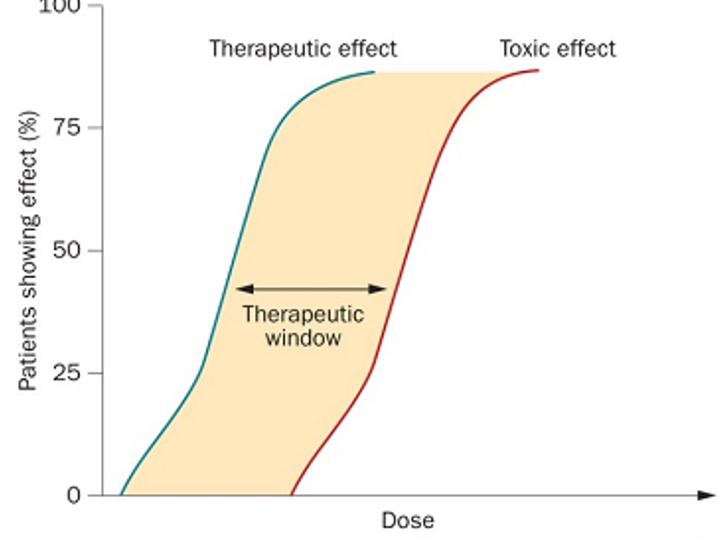
therapeutic window
The therapeutic window is the range of dosages between the amounts of the drug that produce the therapeutic effect without causing toxic effect.
Tolerance
reduction in body’s response to drug due to increased metabolism, need for progressively higher doses to obtain the therapeutic effect
Addiction
compulsive desire of user to take drug regardless of resulting health problems
Bioavailability
Bioavailability is the fraction of the administered dosage that reaches the target part of the human body.
which method of administration has the highest bioavailability?
Intravenous injection has highest (100%)
other drug administration is decreased due to incomplete absorption, decomposition, physiological differences in patients
Drug receptor interactions (3)
o Drugs bind with enzymes or cellular receptors, inhibiting enzymes and activating cellular receptors to alter its state or allow specific molecules to pass through the cell membrane
o Functional groups of drug and receptor should be complementary (have correct orientations) to form dipole-dipole interactions, h-bonds, ionic bonds
o Alkyl (CH3) and phenyl groups interact with non-polar receptor groups via LDF
Discussion of experimental foundations for therapeutic index and therapeutic window through both animal and human studies.
o Use of double blind test to prevent bias in interpretation of experimental results, real vs placebo
o The difference in results is attributed to the pharmacological action of the drug
Ethical considerations for drug development
negative side-effects of medication on patient
potential for abuse
animal testing
risk to benefit ratio
appropriate consent of patient volunteers
side effects
non beneficial effects on the human body
side effects of aspirin
gastrointestinal bleeding
Drug administration based on polarity
o Drugs with polar groups are generally water soluble and are administered orally
o Non-polar drugs administered transdermally (applied on skin in patches, ointments)
what is the repeated risk of using opioids
easily addictive, subject to abuse
2 drug administration routes for drugs unstable in acidity
administered rectally (suppositories or enemas)
administered parentally
into skin (subcutaneous injection)
into muscle tissue (intramuscular injection)
into bloodstream (intravenous injection)
drug administration of volatile drugs
o Volatile or highly dispersed drugs are administered through inhalation (nose or mouth)
Comparison of how functional groups, polarity and medicinal administration can affect bioavailability
o Intravenous injection produces the highest bioavailability as the drug is distributed around the body with the flow of the blood (100%)
o Polar molecules (hydroxyl, carboxyl, animo groups) are soluble in water, quickly absorbed from GI tract into bloodstream but cannot pass through hydrophobic cell membrane thus reducing biological activity
o Non-polar molecules are insoluble in the bloodstream
Placebo effect
administering biologically inert substance to assist body in its natural healing process
Mild analgesics
Mild analgesics (pain killer) function by intercepting the pain stimulus at the source (bind to cyclooxygenase enzyme), often by interfering with the production of substances that cause pain, swelling or fever (prostaglandins)
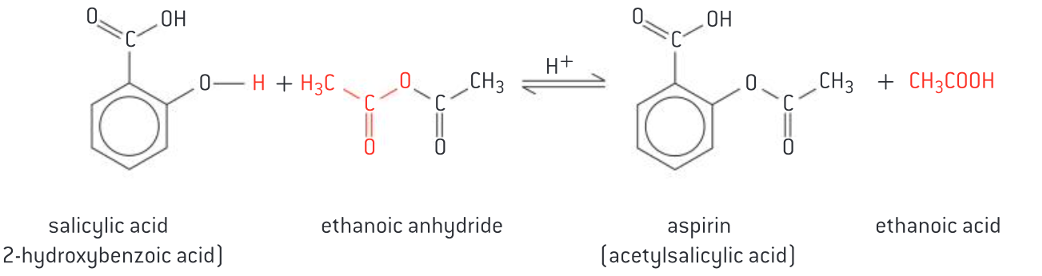
Production of aspirin from salicylic acid
use of ethanoic anhydride to add ethanoic acid to phenyl-bound hydroxyl group
Functions of aspirin
mild analgesic (pain killer)
blood thinner / anticoagulant to prevent heart attacks
Anticlotting actions can lead to excessive bleeding and ulceration of stomach
Why use ASA (aspirin) over pure SA
Pure SA caused severe digestive problems like stomach irritation, can be reduced through acetylsalicylic acid
Explanation of the synthesis of aspirin from salicylic acid, including yield, purity by recrystallization and characterization using IR and melting point. (4)
o SA is mixed with excess ethanoic anhydride and concentrated phosphoric acid as catalyst
o Mixture is heated for short time, then diluted with water and allowed to cool down, producing crystals of aspirin
o Impure product is recrystallized from hot ethanol
o Identity of product can be confirmed through IR spectroscopy or melting point
use of cold water to purify aspirin
Purified through rinsing with cold water as aspirin is less soluble in cold water, [to avoid dissolving the crystals]
Discussion of the synergistic effects of aspirin and alcohol
Aspirin and alcohol have a synergistic effect as alcohol is also an anticoagulant
Soluble aspirin
produced by neutralizing the carboxyl group with NaOH / base, producing COO-Na+ (OH- —> O-Na+)
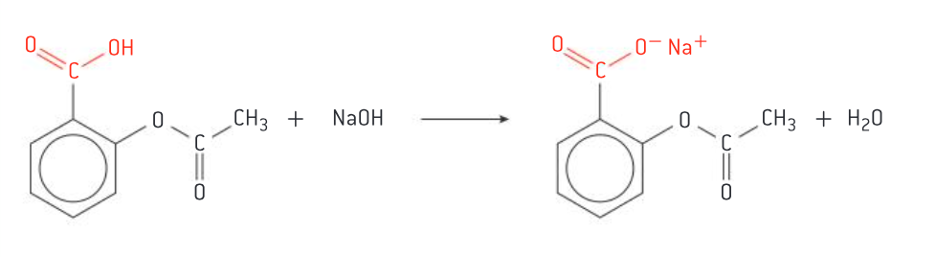
Discussion of how the aspirin can be chemically modified into a salt to increase its aqueous solubility and how this facilitates its bioavailability. (4)
o Aspirin is insoluble in water
o Its aqueous solubility can be increased through neutralizing the carboxyl group with NaOH, producing “soluble aspirin”
o Sodium salt is neutralized with HCl in stomach acid
bioavailability of soluble aspirin is only slightly higher than regular due to solubility
Discuss the impact of aspirin on the pH of blood
Lowers pH as aspirin is a weak acid (acetylsalicylic acid)
Explanation of the importance of the beta-lactam ring on the action of penicillin. (3)
o Beta-lactam ring has bond angles of 90 degrees, creating significant ring strain, making amide groups attached to beta lactam ring very reactive
o Beta-lactam ring irreversibly binds to transpeptidase, responsible for crosslinking in bacterial cell walls, making bacteria more permeable to water
o Osmotic pressure causes bacterial lysis
Why are human cells not affected by penicillin
Humans have animal cells, which do not have cell walls and are not affected by penicillin
Discussion of the importance of patient compliance and the effects of the overprescription of penicillin. (2)
o If patients stop taking medications after symptoms of disease disappear, this allows resistant bacteria to survive and pass resistance to next generation
o Overprescription of penicillin made pencillin-resistant bacteria the dominant species as only those with resistance survived and passed on their genes
Discussion of the effects of chemically modifying the side-chain of penicillins. (2)
o Penicillin side chains were modified to overcome increased production of penicillinase (antibiotic resistance), by modifying side chains, drug could not be deactivated by penicillinase
o Increased stability in acidity of stomach, could be administered orally
Why is codeine less potent than morphine (2)
Codeine readily crosses the BBB but does not bind to opioid receptors due to steric effect of ether group
Codeine is slowly metabolized into morphine causing therapeutic effect, slower reacting therefore less chance of abuse
Strong analgesics
Strong analgesics are used to relieve severe pain, they work by temporarily bonding to opioid receptors in the brain, preventing the transmission of pain impulses without depressing the central nervous system.
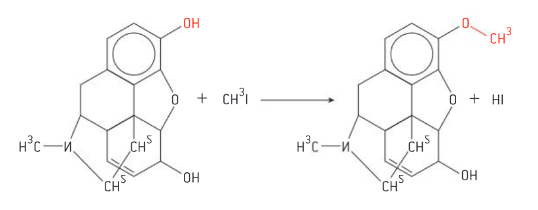
Synthesis of codeine from morphine
replace phenolic OH with less polar ether OCH3
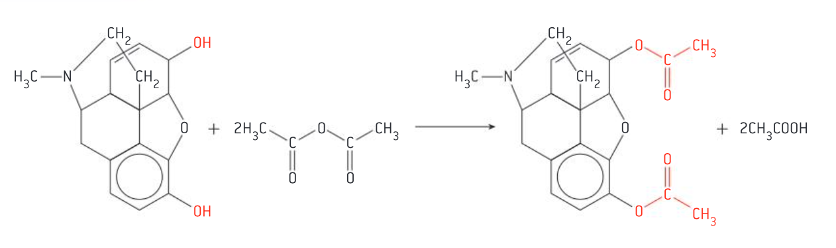
Synthesis of diamorphine from morphine
both OH groups substituted with ester groups (COOH-CH3) using ethanoic anhydride
Advantages and disadvantages of using opioids
o Advantage: relieve severe pain
o Disadvantages: highly addictive, high risk of abuse from strong feeling of euphoria
Short term side effects of opioids (5)
decreased breathing and heart rate, nausea, coma, death in high doses
Long term side effects of opioids (3)
constipation, poor appetite, loss of sex drive
Withdrawal symptoms of opioids (4)
perspiration, diarrhoea, cramps, distress
Explanation of the increased potency of diamorphine compared to morphine based on their chemical structure and solubility. (3)
o Diamorphine is more potent than diamorphine due to its substitution of hydroxyl groups for esters from ethanoic anhydride, making it more lipid soluble
o It quickly metabolizes into morphine, binding to the opioid receptors quicker and making it 5x more potent than morphine
o Has more side effects: tolerance, addiction, CNS depression
Active metabolites
Active metabolites are the active forms of a drug after it has been processed by the body.
How do Prilosec and Nexium achieve the same therapeutic effect despite being enantiomers
both produce the same active metabolites in acidic environment, thus both binding to proton pump enzymes
Neutralization of excess acidity in stomach equations
Al(OH)3 (s) + 3HCl (aq) → AlCl3 (aq) + 3H2O
NaHCO3 + HCl → NaCl + CO2 + H2O
Explanation of how compounds such as ranitidine (Zantac) can be used to inhibit stomach acid production.
Zantac binds to H2-histamine receptors in the cells of gastric lining, reducing the secretion of stomach acid by inhibiting histamine from binding
Explanation of how compounds like omeprazole (Prilosec) and esomeprazole (Nexium) can be used to suppress acid secretion in the stomach.
Prilosec and Nexium inhibit the gastric proton pump enzyme, which is responsible for secreting H+ ions into gastric juice, longer effect than Zantac
4 stages of virus replication targeted by antiviral drugs
Attachment of virus to the host cell
Uncoating of the virus and injection of viral DNA/RNA into the cell
Biosynthesis of viral components by the cell machinery
Release of viruses from cells
Tamiflu and Relenza
Viruses vs bacteria
Viruses lack a cell structure / wall and have no metabolism
Explanation of how oseltamivir (Tamiflu) and zanamivir (Relenza) work as a preventative agent against flu viruses.
Prevent the release of virus copies from infected cell by inhibiting viral neuramindases enzymes, responsible for allowing viruses to pass through cell membrane. By inhibiting them, slows virus spread around body
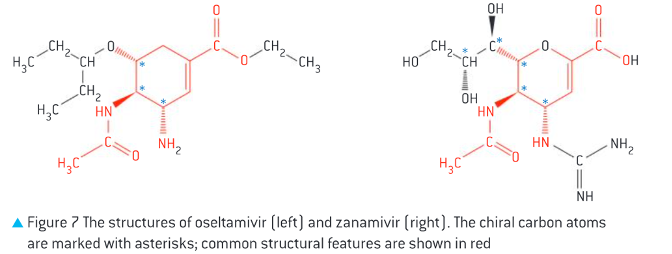
Comparison of the structures of oseltamivir and zanamivir. (3)
o Both have 6 membered ring with 3 chiral carbon atoms but different side groups
Oseltamivir (Tamiflu) has an ester group, making in inactive, it is hydrolysed into a carboxyl group in the body, producing an active metabolite with enhanced antiviral activity
Zanamivir: already has a carboxyl group so it is active in its original form
Discussion of the difficulties associated with solving the AIDS problem. (3)
o HIV has fast replication cycle and high mutation rate, 1010 new copies per day with several modifications
o HIV infects (b cell lymphocytes) white blood cells which are responsible for fighting viral/bacterial infections
o HIV incorporates itself into the host DNA where it can remain dormant for years
High-level waste (HLW)
High-level waste (HLW) is waste that gives off large amounts of ionizing radiation for a long time. ex. nuclear fission products
Low-level waste (LLW)
Low-level waste (LLW) is waste that gives off small amounts of ionizing radiation for a short time. ex. contaminated hospital equipment
disposal of LLW
must be stored for weeks in shielded containers until safe level of radiation, then incinerated or buried shallow underground
disposal of HLW
encased in steel cylinders covered with concrete, buried deep underground in geologically stable locations
Which isotopes are exceptions to LLW disposal guidelines
Co-60 and Cs-137
Impact of ionizing radiation on humans (3)
Ionizing radiation causes cellular and genetic damage (errors in DNA sequences, breaks bonds in DNA)
Low doses increase number of mutations and probability of developing diseases like cancer, birth defects
Weakens immune system by triggering apoptosis in lymphocytes and bone marrow cells therefore more likely to contract infectious diseases
risk of radioactive and antibiotic waste
Radioactive materials and antibiotic waste increase the mutation rate in bacteria, accelerating development of drug-resistant strains
Discussion of environmental issues related to left-over organic solvents.
o Most Organic solvents are toxic to living organisms, affecting the nervous and respiratory systems, organs, reproductive organs
their vapours contribute to greenhouse effect
Chlorinated solvents
Chlorinated solvents are ozone-depleting agents due to their low bond enthalpies and contribute to the formation of photochemical smog in cites
Environmental issues associated with chlorinated solvents (2)
Have limited biodegradability and may accumulate in ground water, causing long-term damage to local ecosystems
Cannot be incinerated with organic waste as their incomplete combustion can produce highly toxic phosgene (COCl2) and dioxins
proper disposal of chlorinated solvents
Disposed by oxidizing separately at very high temperatures or recycled by distillation
Dangers of antibiotic waste
Antibiotics in agriculture leads to environmental pollution, spreading antibiotic resistance due to consumption of animal products
Development of antibiotic resistance from waste and prescription (2)
o Overprescription of antibiotics result in antibiotic resistance as only resistant bacteria reproduce
o Constant exposure to low levels of antibiotics from environmental waste leads to antibiotic resistance
Green chemistry
o Green chemistry: reduce the environmental impact of technological processes by minimizing the use and generation of hazardous chemicals
Such as aqueous or solvent-free reactions, renewable staring materials, mild reaction conditions, regio- and stereoselective catalysis, utilization of by-products formed during the synthesis
Atom economy
efficiency of a synthetic procedure as the ratio between the molecular mass of the isolated target product and combined molar masses of all starting materials, catalysts, and solvents (product over “reactants” [all starting materials])
Advantages of using bioengineering in organic synthesis (3)
enzyme-catalyszed biochemical reactions are highly selective, efficient, proceed in aqueous solutions under mild conditions
Explanation of how bioengineering was used to develop the precursor for Tamiflu (oseltamivir). (2)
o Shikimic acid is a precursor to Tamiflu, which was produced from renewable materials by genetically modified organisms using bioengineering
E.coli is genetically modified, undergoes fermentation which produces shikimic acid, which then can be extracted
Explanation of how shikimic acid can be sourced non-naturally
Shikimic acid can also be formed by readily available cyclic esters (lactone), which forms the correct stereoisomer of oseltamivir in a shorter number of chemical steps than the typical production
Taxol (paclitaxel)
Taxol is a drug that is commonly used to treat several different forms of cancer.
Chiral auxiliary
A chiral auxiliary is an optically active substance that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of a single enantiomer.
readily available chiral reagents that can be temporarily introduced to the starting material and removed when the synthesis is complete
Why is Taxol hard to synthesize synthetically
Taxol has 11 chiral centres
Explanation of extraction of taxol from natural sources using multi-step liquid-liquid partitioning. (4)
Taxol is partitioned between two immiscible solvents, in which Taxol is more soluble in one than the other.
This process is repeated multiple times to increase yield.
The solvent is evaporated from the final solution to produce pure Taxol.
effectiveness of each solution can be tested using degree of tumour inhibition in lab animals ( T/C = mean tumour mass in treated / control animals x100 )
Taxol solubility
almost insoluble in water so unsuitable for intravenous administration
Description of the use of chiral auxiliaries to form the desired enantiomer. (3)
Chiral auxiliary is added to reaction, creating the stereochemical condition necessary to follow a certain pathway as…
An existing chiral centre in the molecule affects the configurations of newly formed chiral centres
Chiral auxiliary is removed, producing the preferred enantiomer

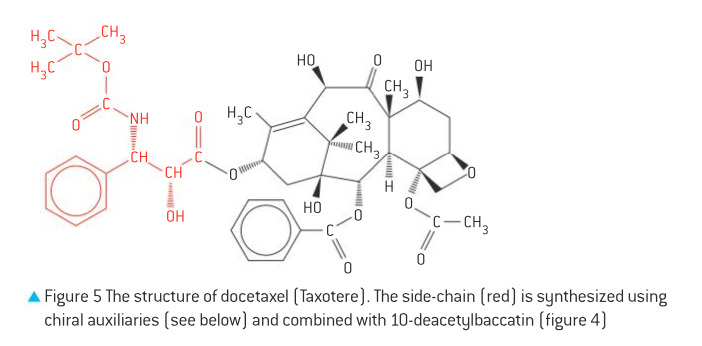
Docetaxel (Taxotere)
more active than Taxol, soluble in water so suitable for IV injection
derived from 10-deacetylbaccatin, from European yew leaves with 50x higher yield
Polarimeter
measures the angle of rotation of plane-polarized light caused by optically active molecules
Use of polarimeter to identify enantiomers (4)
o Under identical conditions, two enantiomers of the same compound will rotate light by the same angle but in opposite directions
o Each isomer has their own unique rotation angle
o A mixture of two enantiomers (50/50) will be optically inactive, angle of 0
o Changes in the rotation angle of a known substance indicates impurities
types of ionizing radiation (3)
Alpha (24He / a) particles have two protons and two neutrons
beta (b- or e-) are high energy electrons
gamma rays are photons with short wavelengths (high frequency)
Alpha radiation isotopes
Pb-212 (Lead), Ac-225 (Actinium)
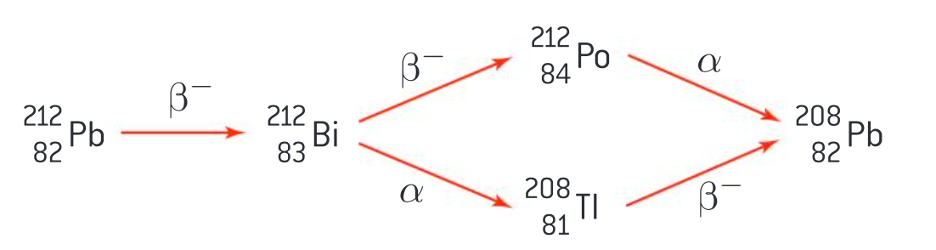
Lead-212 decay chain (2 routes)
both alpha and beta decomposition
beta, alpha, beta
beta, beta, alpha
beta radiation isotopes
Lu-177 (Lutetium), Y-90 (Yttrium), I-131 (iodine)
Gamma radiation isotopes
Tc-99m (Technetium), I-131
Why is MRI more accurate imaging than others
MRI provides more detailed images as the protons in water, lipids, carbohydrates have different chemical environments, distinguished by H NMR
Brachytherapy (internal radiotherapy)
radiation sources are inserted into the patient’s body in metal wires/pellets to deliver radiation
External radiotherapy
External radiotherapy involves precisely directed beams of radiation to destroy cancer
Properties of alpha particles for cancer treatment (3)
Alpha particles have high ionizing density, so cause more damage to cellular tissues than other forms of radiation,
however they have low penetrating power and are completely absorbed within a short range of their emission (0.05-0.1mm)
used for treating leukaemia and dispersed cancers
properties of low-energy beta particles vs alpha particles
low energy beta-particles have a reduced tissue penetration compared to alpha particles
Boron Neutron Capture Therapy (BNCT)
o Boron neutron capture therapy uses boron-10 to absorb a neutron, resulting in boron-11 which undergoes alpha decay
Boron 10 can be absorbed by specific tissues
- used to treat brain, head, neck cancers
short term side effects of radiotherapy (4)
hair loss
nausea
fatigue
sterility.
Long term risk of radiotherapy
development of secondary cancers
Explanation of why technetium-99m is the most common radioisotope used in nuclear medicine based on its half-life, emission type and chemistry. (5)
o Tc-99m emits gamma radiation, ideal for x-ray visualization
Photons emitted by Tc-99m have approximately same wavelength as x-rays, easily detected by x-ray equipment
o Energy of photons is low, reducing radiation received by patient
o Tc has various stable oxidation states, readily forms complexes with various ligands, which can be administered by injection to specific organs/tissues
o Tc-99m has a half-life of 6 hours, making it ideal for medical imaging, all administered Tc decays within 2 days within patient
o Half-life is long enough to prepare complexes with biologically active ligands
Explanation of why lutetium-177 and yttrium-90 are common isotopes used for radiotherapy based on the type of radiation emitted.
o Produce beta radiation, low-energy beta particles have reduced tissue penetration, which is useful for targeted therapy of small tumours
o Also emits enough gamma rays for tumour visualization
Alpha decay
loss of two protons and two neutrons, mass number reduced by 4, atomic number reduced by 2
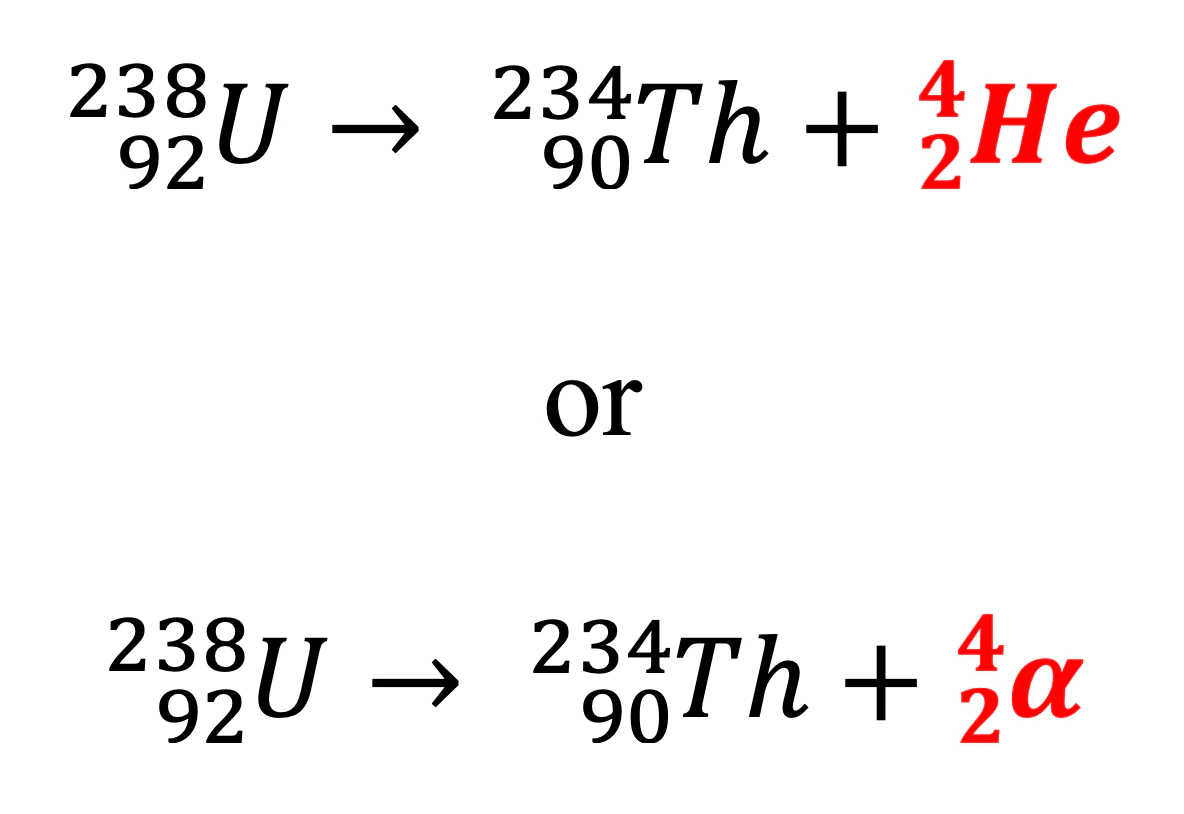
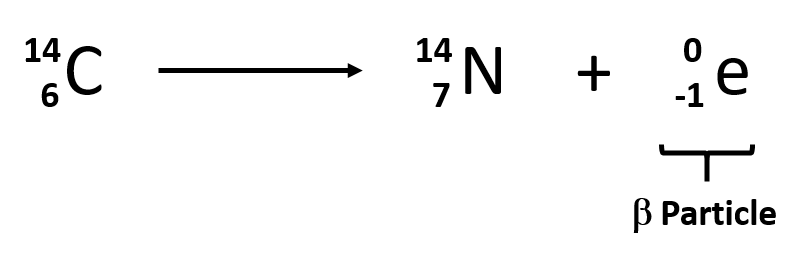
Beta decay
neutron decays into proton and an electron, release of e- while keeping mass number the same, atomic number increases by 1
Half life equation for radioactive decay