Chemistry 3.1- Introduction to Organic Chemistry
1/25
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Alcohols: functional group, prefix/suffix
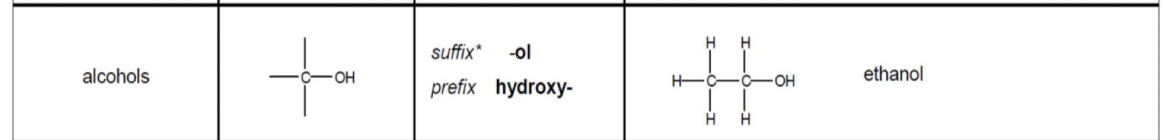
Aldehydes: functional group, prefix/suffix

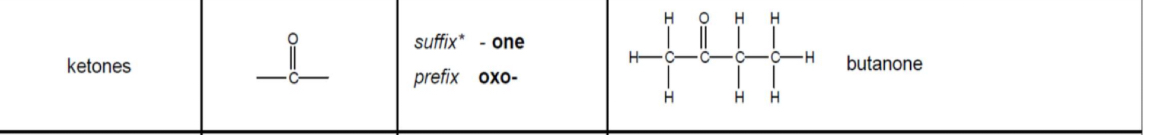
Ketones: functional group, prefix/suffix
Nitriles: functional group, prefix/suffix
Amines: functional group, prefix/suffix

What is the functional group priority order?
Carboxylic acids > nitriles > aldehydes > ketones > alcohols > amines > alkenes > haloalkenes
What are the types of isomerism?
Structural: where two or more compounds have the same molecular formulas but different structures
Chain
Positional
Functional Group
Stereoisomers: where two or more compounds have the same structural formula but different spatial arrangements
Geometric
Optical
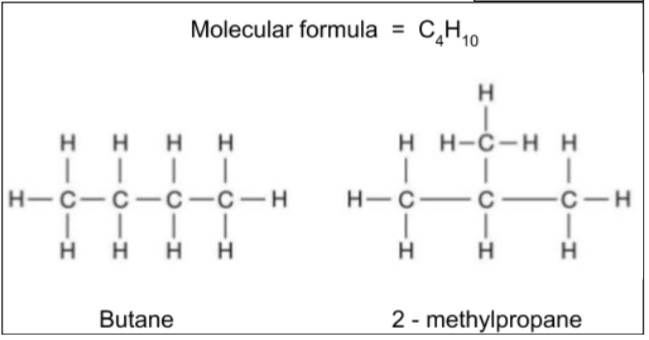
Chain isomerism
Different arrangements of the carbon ‘skeleton’, changing whether there are branched chains
Position isomerism
Moving the functional group to a different position in the carbon chain
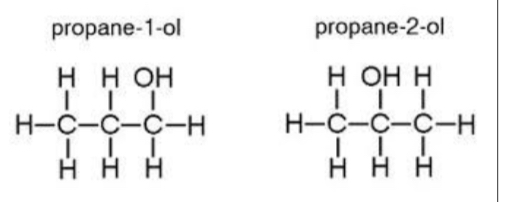
Functional group isomerism
Changing the functional group of the compound, keeping the molecular formula the same
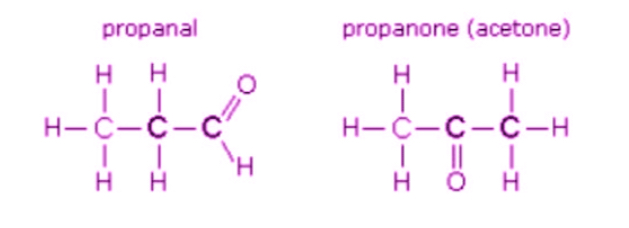
Geometric isomerism
Same bonding in the structure, but a different spatial arrangement in planar (2D) molecules, due to restricted movement around a C-C double bond
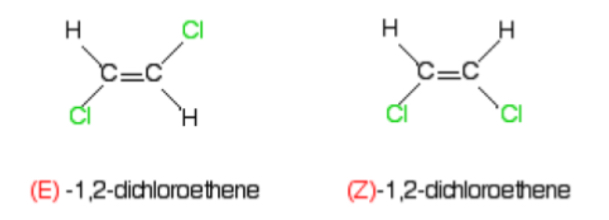
Groups are either trans (on opposite sides) or cis (on the same side) if they are the same group
Groups are either E (on opposite sides) or Z (on the same side) whether they are the same group or different
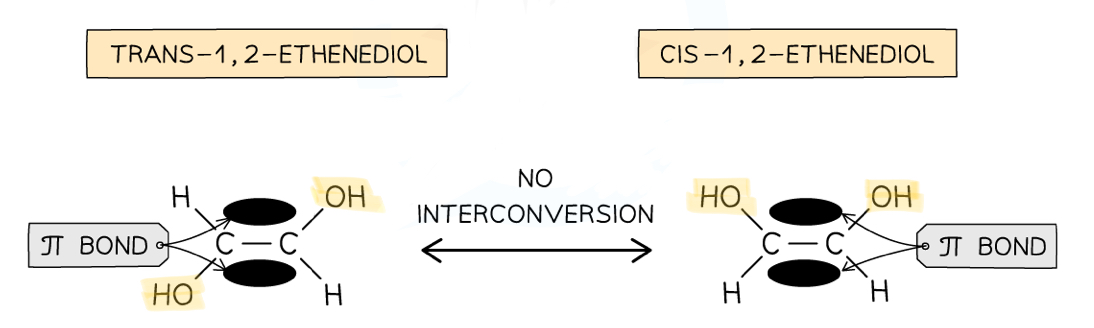
Why does geometric isomerism occur in some compounds with a C=C double bond?
Double covalent bonds are made up of one sigma bond and one π bond
This π bond restricts rotation of the carbons, so the spatial arrangement of attached groups can’t change
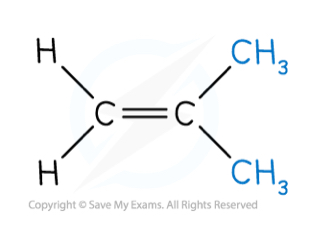
Does this compound have geometric isomerism? If so, what are the two isomers?
The pair of groups on either carbon are the same, so it has no geometric isomerism
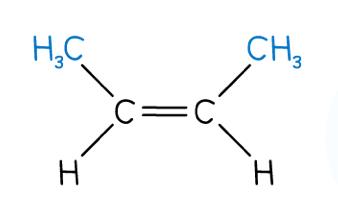
Does this compound have have geometric isomerism? If so, what are the two isomers?
The pair of groups on each carbon are different, so yes, it has geometric cis/trans and E/Z isomerism
The cis isomer is also a Z isomer, and the trans isomer is also an E isomer

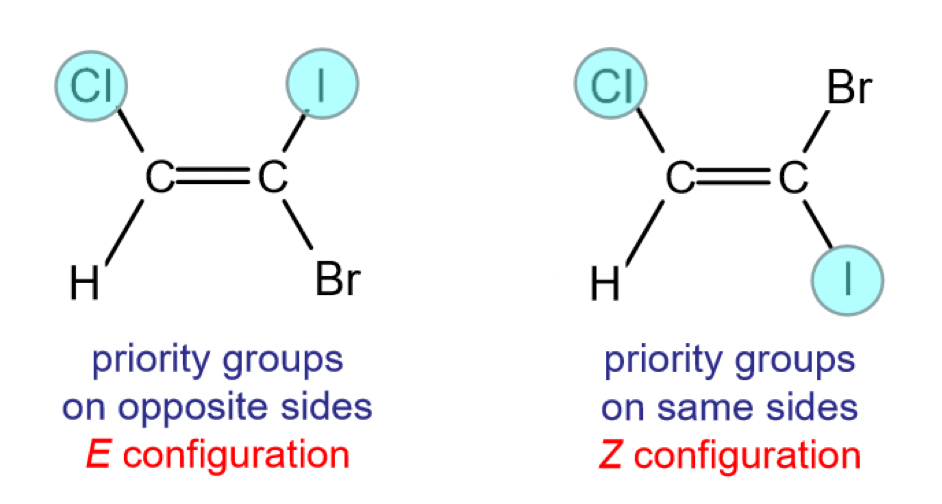
How do we use E/Z isomerism?
Following CIP priority rules
Priority is given to the group with the higher atomic number on each carbon in the C-C bond
Eg. If the two groups are OH and CH3, the OH will be the priority group, as oxygen has a higher atomic number than carbon
E isomers have the two priority groups on opposite sides, and Z isomers have them on the same side
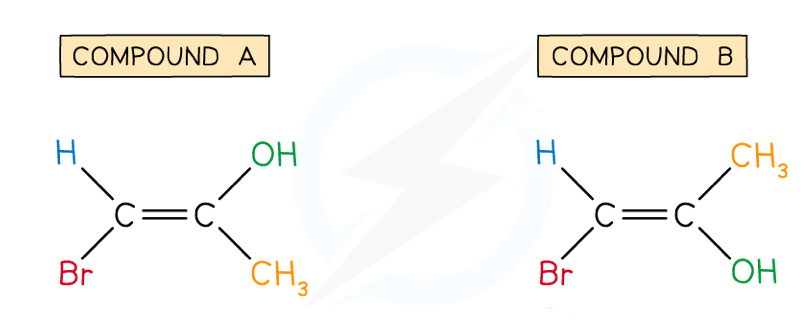
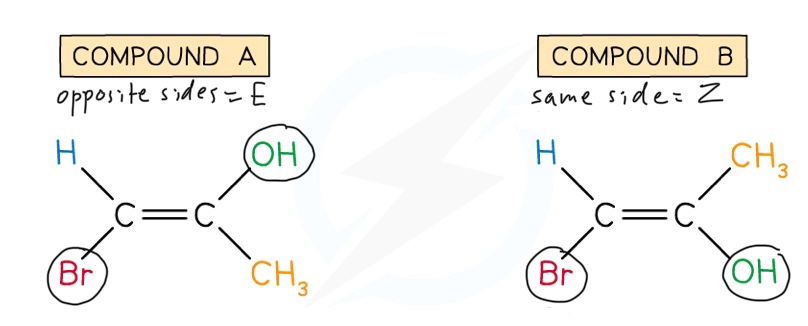
Label these compounds as E and Z isomers
Br has priority over H and OH has priority over CH3 (priority is given to groups with higher atomic numbers), so:
Compound A would be the E isomer
The Br is on the opposite side to the OH, so it is the E isomer
Compound B would be the Z isomer:
The Br is on the same side as the OH, so it is the Z isomer
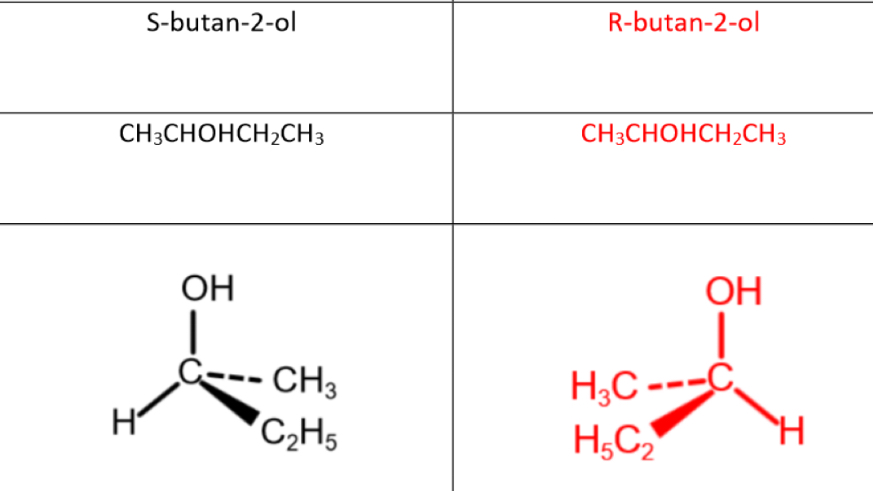
Optical isomerism
Same bonding in the structure, but a different spatial arrangement in non-planar (3D) molecules- mirror images
Groups are either S (left) or R (right)
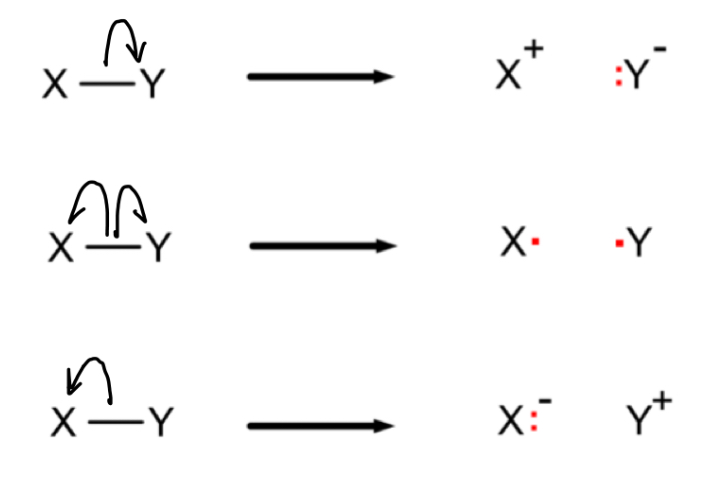
What is a homolytic fission mechanism?
Breaking a covalent bond to give each atom an electron, forming two free radicals
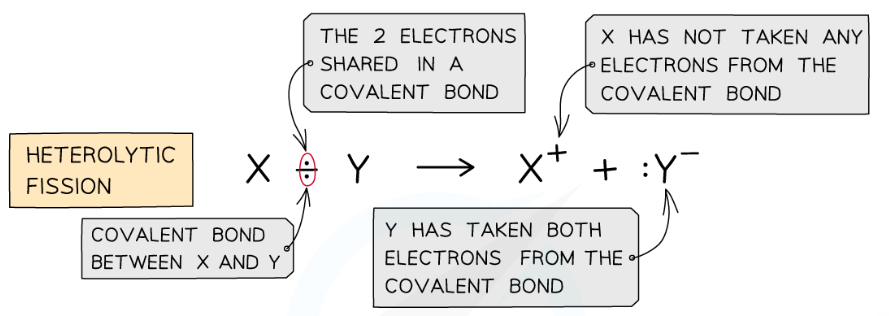
What is a heterolytic fission mechanism?
Breaking a covalent bond to give the more electronegative atom both electrons, forming a negative ion and a positive ion
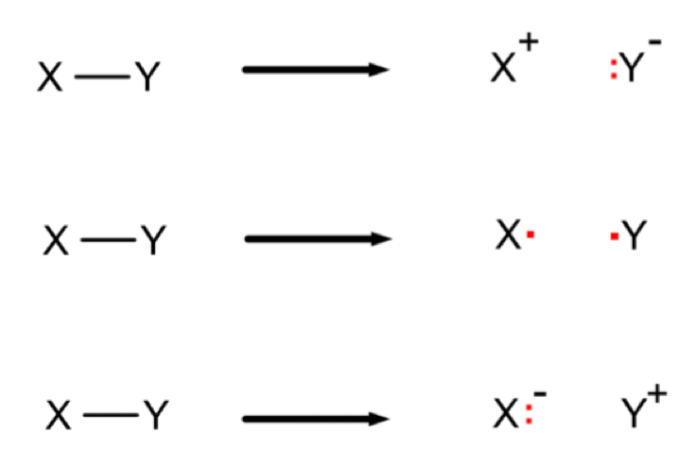
How would you draw the mechanism for each of these examples of bond fission?
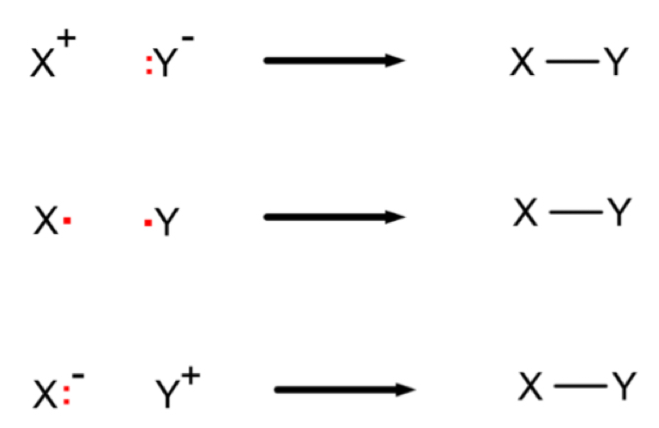
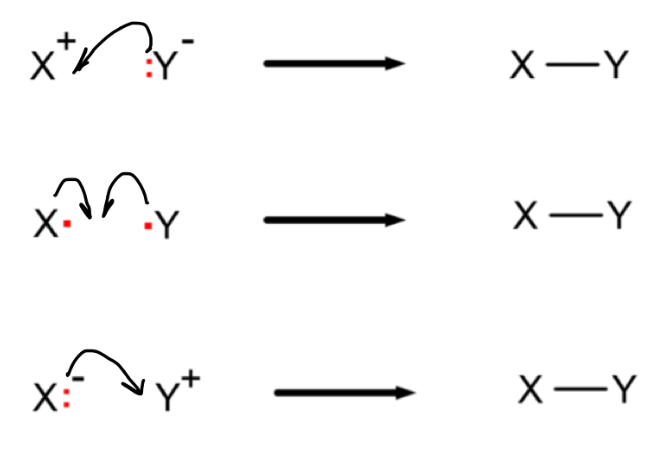
How should you add draw the mechanism for each of these examples of bond formation?
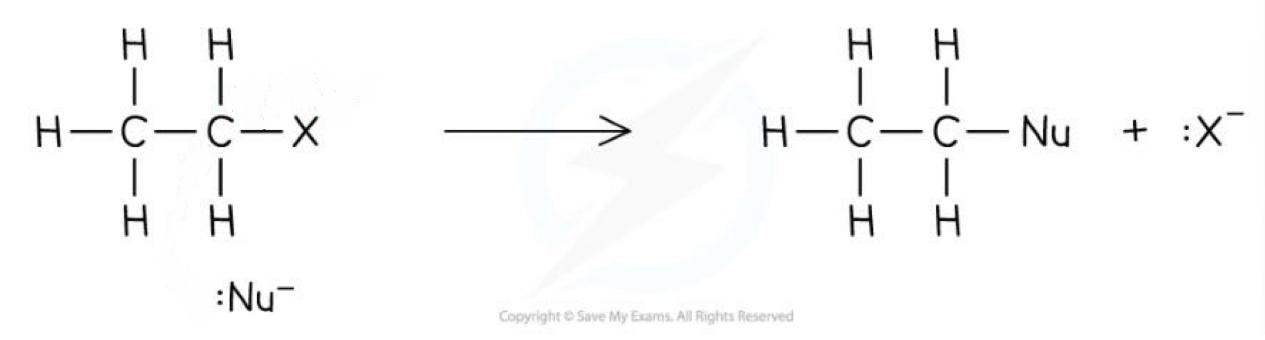
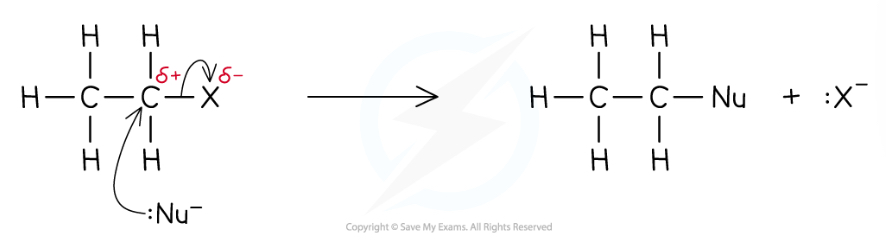
How should you draw this mechanism? What kind of mechanism is it?
Nucleophilic substitution
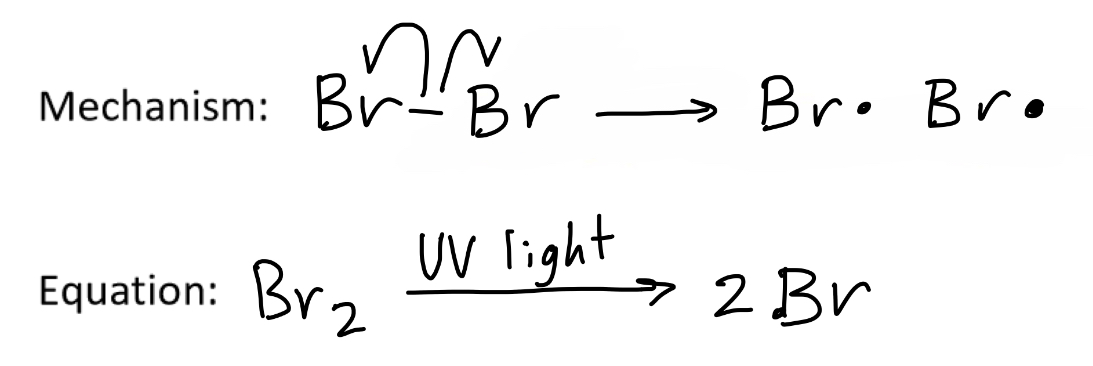
For a free radical substitution involving bromine, what is the mechanism and equation of the initiation step?
For a free radical substitution of bromine into methane, what are the equations of the propagation steps and overall reaction?
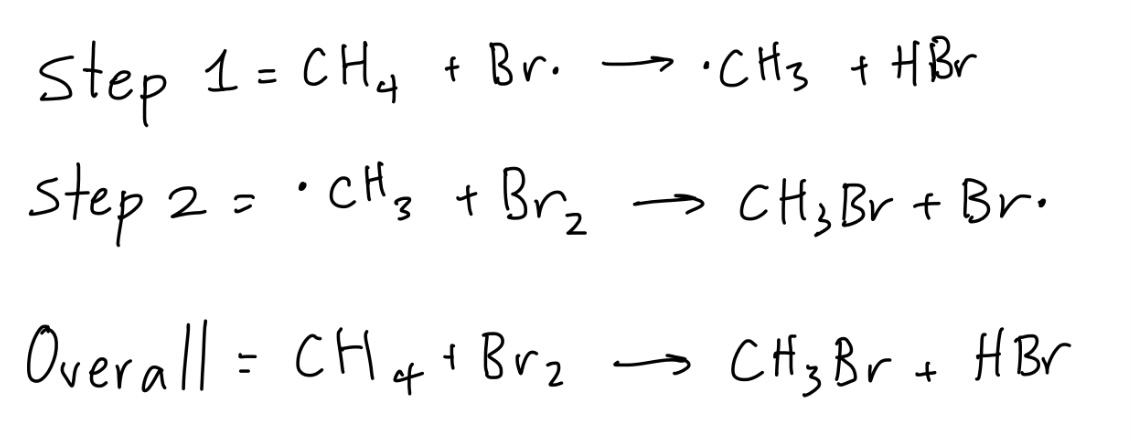
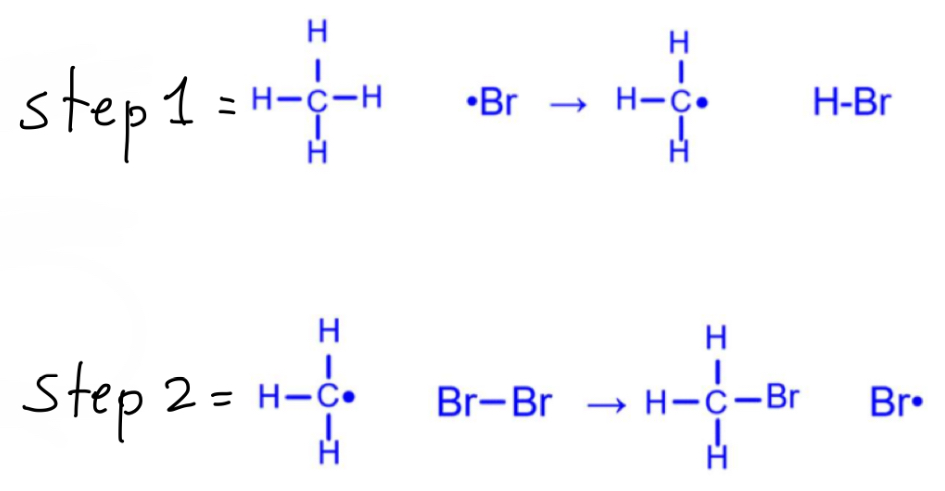
How should you draw these propagation step mechanisms?
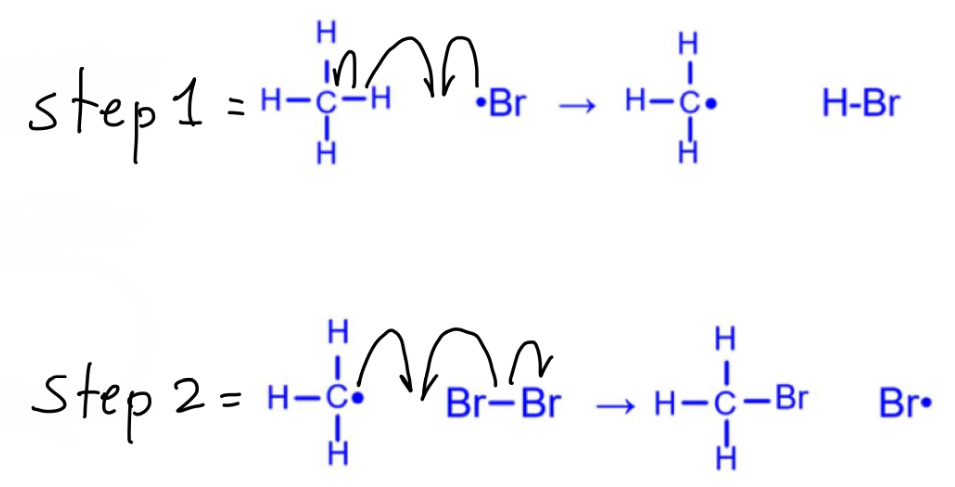
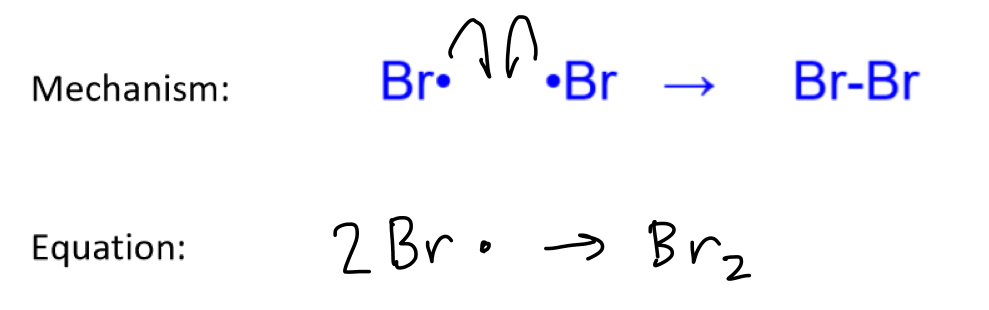
For a free radical substitution, what is the mechanism and equation of the termination step to produce Br2?