NP Excitation modulation of membrane potential
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What modulates resting membrane potential in neurons?
Synaptic inputs modulate resting membrane potential to take it closer to threshold through depolarization/excitation or further away through hyperpolarization/inhibition. This leads to changes in graded membrane potential.
What are the three classes of neurotransmitter?
Small-Molecule Neurotransmitters
Examples
Purines: e.g. ATP, Amino Acids: Glutamate, GABA, Glycine.
Biogenic Amine Neurotransmitters
Examples
Biogenic Amines: Catecholamines (Dopamine, Norepinephrine, Epinephrine), Indoleamine (Serotonin), Histamine.
Peptide Neurotransmitters
Serve specific functions in neurotransmission, work via metabotropic receptors
What types of synaptic inputs are there that can modulate membrane potential?
Synaptic inputs can be mediated by chemical or electrical connections
Chemical synapse
Majority of synapses
Release a chemical neurotransmitter (from vesicles into synaptic space) in response to an action potential
Electrical synapse
Gap junctions enable direct ionic passage between neurons, altering postsynaptic potentials.
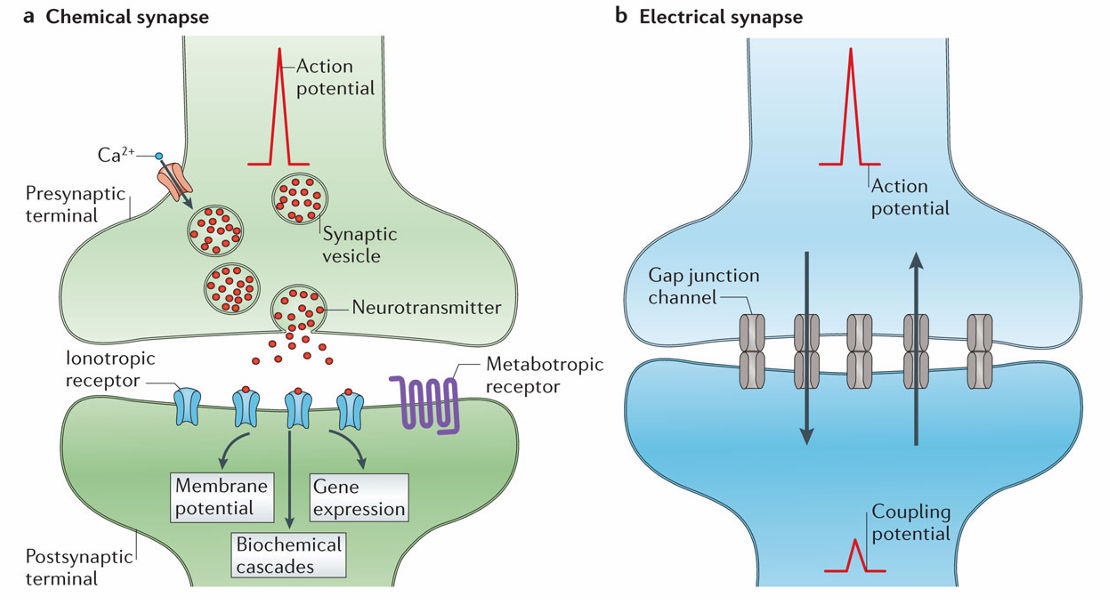
Identify and describe the two types of neurotransmitter receptors
Ligand-Gated Ion Channels (Faster, Ionotropic Receptors)
Neurotransmitter binding opens ion channels, allowing ion movement across the membrane.
The channel will have selectivity but the direction of flow of ions will depend on the electrochemical gradient
These are allow neurotransmitters to be fast as they are almost like an on/off switch
G-Protein-Coupled Receptors (Slower, Metabotropic Receptors)
Neurotransmitter binding to the receptor activates G-proteins, modulating ion channels
Most neurotransmitters work via both ionotropic and metabotropic receptors but metabotropic receptors are slower and allow for modulation
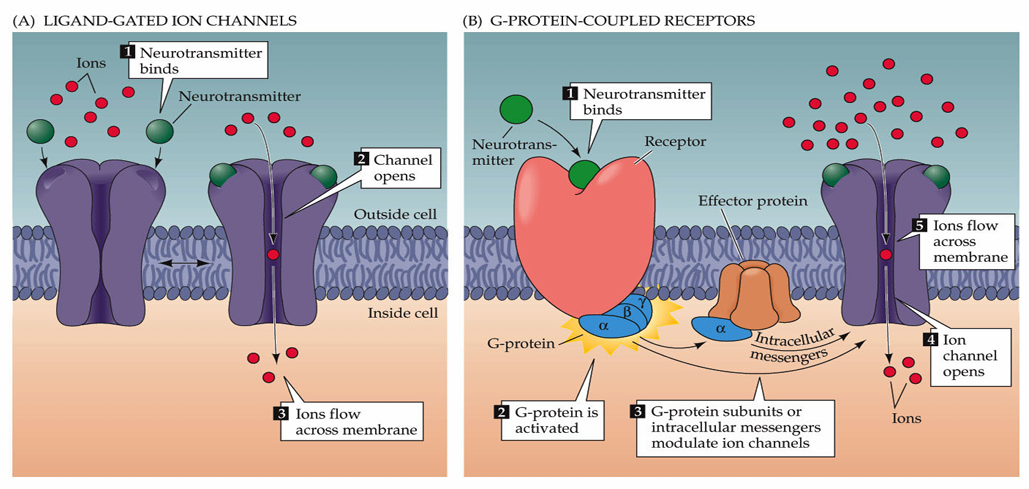
What is the principal fast excitatory neurotransmitter within the mammalian central nervous system?
Glutamate
What are the three principal glutamate ionotropic receptors?
AMPA, NMDA & Kainate each having different pharmacology and actions
What is the basic structure of glutamate ion channels?
The channels are made up of subunits which have three transmembrane-spanning domains and a re-entrant loop. Most models predict 4 subunits making up an ion channel. There is also an extracellular amino terminus and an intracellular carboxy terminus.
Different receptors are made up of differing combinations of subunits, allowing them to have different characteristics
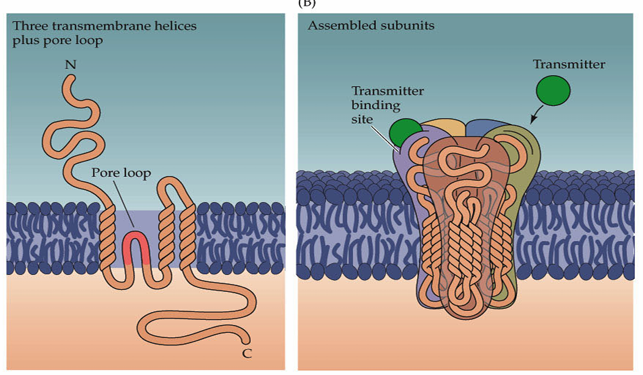
How does the AMPA glutamate receptor work?
Metabotropic receptor - the ion channel opens when the glutamate neurotransmitter binds to the receptor, allowing ions to flow through
This channel is a non selective cation channel, meaning it allows equal flow of sodium and potassium
Sodium will flow into the cell predominantly, because there is a high electrical and chemical gradient for sodium (interior is negatively charged)
This influx of sodium leads to the depolarization of the interior of the cell (less negative)
As a response the excitatory post synaptic current increases
How does the NMDA glutamate receptor work?
Glutamate neurotransmitter binding to the NMDA receptor opens a channel that transmits Ca2+, Na+ and K+
Has a Mg2+ ion that blocks the ion channel so nothing can pass through
Depolarization of the neuron, leads to repulsion of the Mg2+ ion away from the site, so the ion channel can open and ions can flow through
Thus, a NMDA glutamate receptor needs both ligand binding and a change in voltage to allow the flow of ions through the channel
A crucial difference is that the NMDA receptor leads to the increase of intracellular calcium which is critical for many processes
Explain the process of coupled AMPA and NMDA glutamate receptors.
AMPA receptor activation can happen alone without NMDA
NMDA receptor activation requires activation of AMPA first
Glutamate binds to the AMPA receptor, raising the excitatory post synaptic current and depolarisation of the interior of the cell (rapid)
This enables the glutamate binding to the NMDA receptor to open the channel, as well as the change in voltage allowing the Mg2+ to move out of the way (more prolonged), allowing the ion channel to open
How is glutamate neurotransmitter produced in presynaptic terminals?
Glutamine converted to glutamate via glutaminase in presynaptic terminals.
It is then packaged in synaptic vesicles by VGLUT (vesicular glutamate transporter) followed by release at synapses, through fusion of the vesicles with the membrane.
How can glutamatergic neurons maintain high firing rates and fidelity and explain how these processes occur?
Rapidly removal of glutamate from the synaptic cleft, enables high fidelity of the signalling
Glutamate rapidly removed from synaptic cleft by transporters (EATT, excitatory amino acid transporters
The predominant form of EATT is present in the cell membrane of the glial cells, particularly astrocytes
Glutamate can be removed, recycled, repackaged and reused
EATT, excitatory amino acid transporters take the glutamate in to the intracellular space of the astrocyte, where it is inactivated by glutamine synthetase and is recycled to glutamine, which is inactive so can be released into the extracellular space and transported by EATT back to the presynaptic space
Once in the presynaptic terminal, glutamine can then be converted to glutamate by glutaminase, so it can be repackaged and used for another synapse
What fast ionotropic neurotransmitter is used for communication between motor neurons and muscles and what is it’s ionotropic receptor called?
Acetylcholine which uses nicotinic receptors
What is the structure of nicotinic receptors?
They are non-selective cation channels constructed from pentamers of four transmembrane domain subunits.
What is the slow metabotropic receptor used for the neurotransmitter acetylcholine?
Muscarinic receptor (G-coupled protein receptor)
How is acetylcholine cleared from the synaptic cleft?
Degradation. Acetylcholine is rapidly metabolized in the synapse by the enzyme, acetylcholinesterase
In contrast to glutamate, acetylcholine is not rapidly removed from the synaptic cleft by transporters, it is instead broken down by acetylcholinesterase which reduces it to acetate and choline
From here the acetate is broken down and the choline is recycled via the Na+/Choline transporter that facilitates its reuptake into the presynaptic neuron.
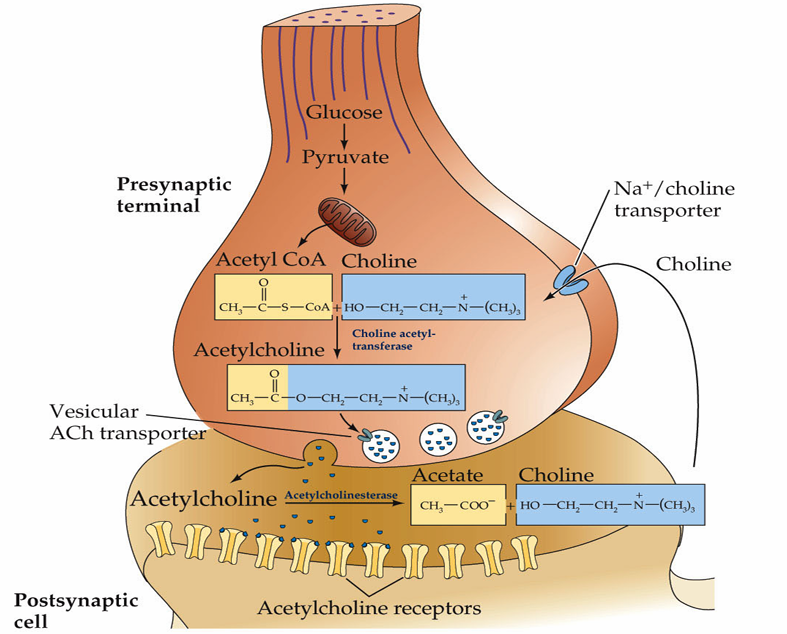
How do electrical synapses work?
Electrical synapses enable ionic currents to pass directly between neurons, influencing membrane potential through gap junctions.
For electrical synapses, there is a physical connection between the intracellular sides of the pre and post synaptic elements, this is via gap junctions called connexins
They can also be modulated to be opened or closed, just because they are open doesn’t mean they always allow for the flow of ions
What is the direction of ion movement in gap junctions?
Ion movement may be bi-directional in gap junctions
An input into one cell, leads to a change in the membrane potential of that cell, thus ions move following the electrochemical gradient into the other cell
These gap junctions can also be used for the movement of other small molecules such as ATP or calcium
Many ionotropic receptor ion channels are not selective for a particular ion and do not determine the direction of ion movement - what does?
The effect of opening the channel is dependent on:
membrane potential
ion concentrations