2 Innate Immune System
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
body's first defense against pathogens
physical, chemical, and biological defense (non-specific)
physical barriers against pathogens
-skin
-GI linings
-genitourinary tract
-Resp. tract
-coughing, sneezing, diarrhea, flushing
chemical barriers against pathogens
-saliva
-tears
-ear wax
-sweat
-mucus (lysozyme)
-peptides
-stomach acid
-proteases
which part of the non-specific defense system is biologic?
GI flora compete for nutrients and change the environment (eg/ pH)
describe innate immunity
-non specific recognition of molecular shapes on pathogens
-always present
what are the major differences between innate and adaptive immune responses?
INNATE:
-no memory
-rapid response (hours)
-fixed immunity
-limited specificity
-constant during response
ADAPTIVE:
-immunologic memory
-slow response (days, weeks)
-variable immunity
-numerous, highly selective specificities to certain antigens on pathogens
-improves during response (ie/ memorizes pathogens so subsequent exposures are faster and stronger)
cells included in the innate immune response
-macrophages
-neutrophils
-eosinophils
-basophils
-monocytes
Innate and adaptive immunity (do/do not) work together
do work together
describe adaptive immunity
-highly specific
-acquired once pathogens evade non-specific physical and chemical barriers and innate immunity
-includes cellular (cytotoxic) and humoral (antibody) defenses
At what point does innate immunity generate a memory?
never generates memory
Functions of the innate immune system
Induce inflammatory response:
-pathogen recognition (seems counterintuitive, but know this one)
-effector cell recruitment
-cytokine production
-complement cascade activation
-foreign substance removal
Activate adaptive immune system
How long is innate immunity present for?
always present
major players in the innate immune system
-phagocytes (neutrophils, macrophages, monocytes)
-mast cells
-basophils
-eosinophils
-NK cells
-complement proteins
-cytokines
Describe neutrophils as they relate to the innate immune system (structure; length of action; relative abundance; when they arrive at the site of infection; primary functions)
-short lived PMN granulocytes
-most abundant leukocyte
-1st cells recruited at infection site
-primarily do phagocytosis, then die
-contain granules w/ toxic substances to kill pathogens
What is a respiratory burst?
An oxidation reaction that occurs in order to kill pathogens that have been recently phagocytized. Generally performed by neutrophils
how are dead neutrophils removed?
phagocytosis by macrophages
what is pus?
dead neutrophils
describe basophils and eosinophils as they relate to the innate immune system (structure; what they defend against and how; how they participate in inflammation)
-PMN granulocytes
-defend against parasites and bacteria
-regulate vascular mediators responsible for inflammation
-responsible for tissue damage during allergic rxns
describe mast cells as they relate to the innate immune system (where they reside; what processes they are associated with; what do they release; what happens when they are activated; when they are activated)
-found in connective tissues and mucus membranes
-associated w/ allergy, anaphylaxis, physical injury, immunologic responses
-granules rich in histamine, heparin, chemokines
-activation leads to degranulation and/or synthesis of lipid-derived chemical mediators involved in inflammation
2 types of phagocytes
-monocytes/ macrophages
-neutrophils
describe macrophages (how long they live; what cell type they're derived from; how they work)
-long lived tissue resident derived from monocytes
-most efficient phagocytes that are able to move outside vasculature and can engulf large numbers of cells and pathogens
what will trigger macrophages, and what do the macrophages do upon being triggered??
-binding of pathogen PAMPs to PRRs on macrophage triggers engulfing of pathogen via respiratory burst
-pathogens also stimulate macrophages to secrete chemokines which attract other immune cells to site of infection
when do macrophages arrive at the site of infection?
>24 hours after neutrophils
describe the process of phagocytic killing (AKA respiratory burst)
1) pathogen binds to and is phagocytosed by neutrophil or macrophage
2) phagosome forms
3) phagosome fuses w/ azurophilic granules and specific granules
4) pH rises, antimicrobial activity ↑, and pathogen dies
5) pH ↓, phagosome fuses w/ lysosomes that secrete more toxic substances to completely degrade pathogen
6) Neutrophil dies via apoptosis and is phagocytosed by macrophage
functions of NK cells
DO NOT directly attack microbes, but instead destroy compromised cells (ie/ virus-infected cells)
why do NK cells require activation in order to kill infected cells?
They don't
what activates NK cells?
IFN-alpha
IFN-beta
what stimulates NK cells to release inflammatory mediators and what are the inflammatory mediators they release?
IL-12 causes production if IFN-gamma and other cytokines
are NK cells involved in the innate or adaptive immune response?
BOTH
where are dendritic cells located and what are their function?
Tissue residents that are phagocytic; mainly found in skin and inner mucosal linings, but also in peripheral organs
function of dendritic cells
-pick up and process degraded pathogens
-antigen-presenting cells that interact with T cells
-serve as link b/w innate and adaptive immune systems
describe the complement system (what comprises it, where are they found)
-composed of plasma proteins C1-C9 (about 30 different proteases made by liver and found in blood, lymph, and extracellular fluids)
-present as inactive proenzymes that are sequentially activated upon infection and kill pathogens
the complement system is a:
biochemical cascade that promotes inflammation and pathogen destruction
4 major functions of complement system
1) lyse bacteria
2) opsonization
3) inflammatory response via triggering histamine release from mast cells
4) clearance of antigen-antibody complexes via ADCC (antibody dependent cell-mediated cytotoxicity)
complement system causes COIL
major cytokines
1) interleukins
2) TNF alpha
3) interferon type 1 (IFN alpha and beta)
4) interferon type 2 (IFN gamma)
who produces interleukins and when?
macrophages and lymphocytes in response to a pathogen or stimulation by other inflammatory mediators
who secretes TNF alpha and when?
-produced by macrophages in response to a pathogen
-increases synthesis of inflammatory proteins
function of TNF alpha
causes muscle wasting, fever, and thrombosis
which IFNs are type 1?
alpha and beta
-where do type 1 IFNs come from?
-what do IFNs do?
secreted by virus-infected cells, which causes an "interferon response" that:
-activate NK cells
-induce resistance to viral replication
-increase expression of ligands for receptors on NK cells
how are IFNs classified?
antiviral
which interferons are type 2?
interferon gamma
where does interferon gamma come from?
made by NK and T cells
remember that IFN alpha and beta stimulate NK cells, so it goes like: IFN alpha and beta → NK cells secrete IFN gamma
function of interferon gamma
increases macrophage microbicidal activity
classic signs of inflammatory response
-redness
-pain and tenderness
-swelling
-fever
what are the benefits of a fever as it relates to the immune system?
-helps immune system fight infection
-slows pathogen replication
-adaptive immune response is more active at higher temperatures
-normal cells are more resistant to effects of TNF alpha at higher temperature
purposes of inflammation
-control infection and prevent further damage
-heal damaged tissues
-initiate adaptive immune response
describe how inflammatory mediators and major cytokines contribute to inflammation (ie/ what are the steps of inflammation)
1) inflammatory mediators are produced by tissue resident effectors upon insult
2) vasodilation and increased vascular permeability
3) recruitment of phagocytes and other inflammatory cells
4) killing of injured or infected cells or damaged tissue cells
inflammatory mediators produced by effector cells
-eicosanoids (prostaglandins and leukotrienes)
-cytokines (ILs, IFNs, TNF, chemokines)
-other toxic substances (histamine, bradykinin, serotonin)
tissue resident effector cells
-macrophages
-dendritic cells
-mast cells
MDM = Most Dense Mofos
where are tissue resident effector cells located?
located in ALL tissues
what chemokines are relevant in inflammation?
Who produces them?
What do they do?
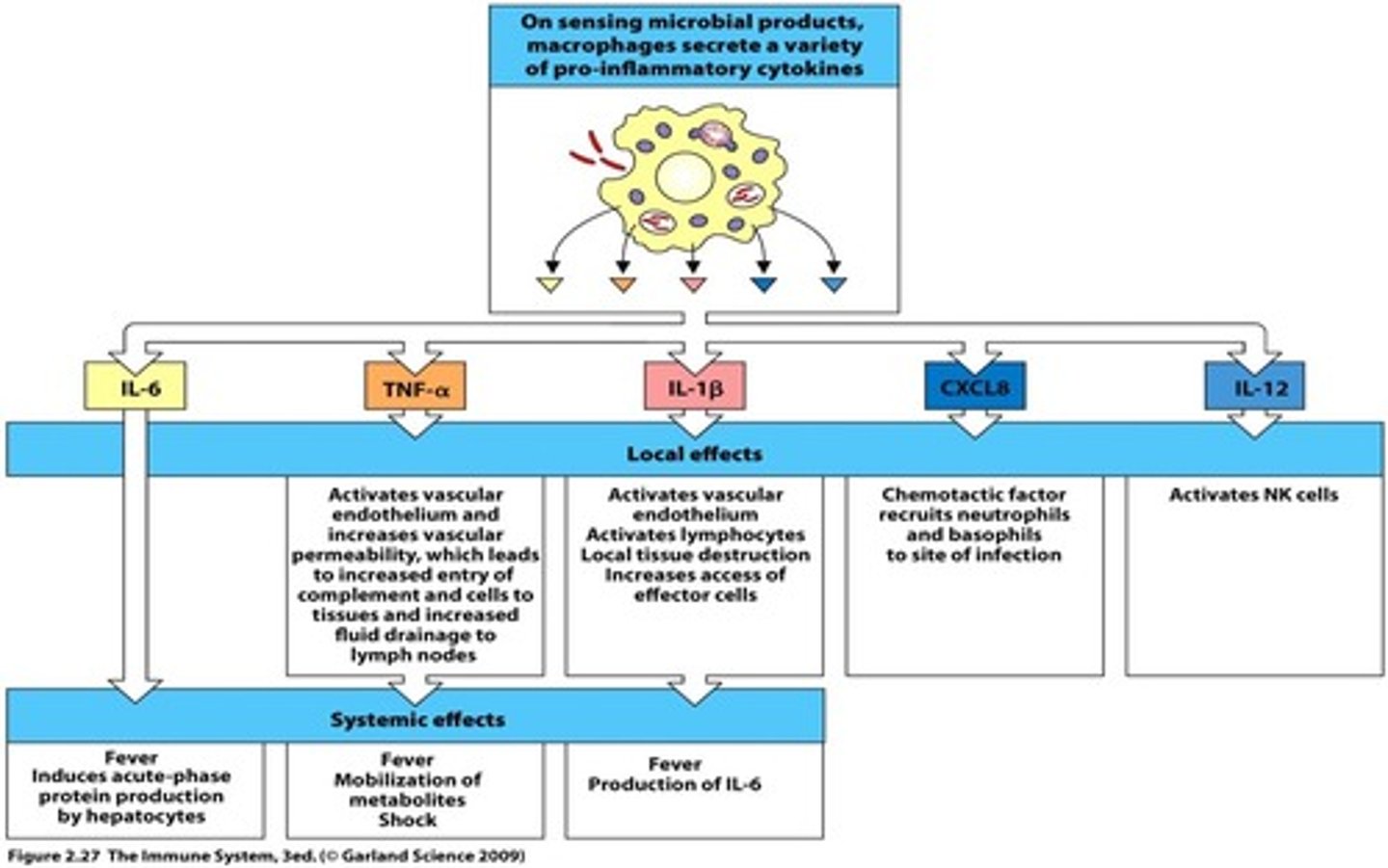
-macrophages produce CXCL8 which bind CXCR1 and CXCR2 to recruit other inflammatory cells
-also produce IL-12 that activates NK cells
which cells perform phagocytosis?
neutrophils and macrophages
describe the process of phagocytosis
1) pathogen is phagocytosed
2) phagosome fuses w/ azurophilic and specific granules
3) pH of phagosome rises, and pathogen is killed
4) pH decreases and lysosomes allow acid hydrolases to completely degrade pathogen
5) If this is a neutrophil, the neutrophil dies and is phagocytosed by a macrophage
consider the complement system and describe these terms:
-lysis
-opsonization
lysis = forming holes via membrane attack complex (MAC), causing pathogen to swell and burst
opsonization = tagging pathogens to enhance phagocytosis
what happens when the complement system activates the inflammatory response?
-increases microbicidal activity of granulocytes (eg/ histamine release from mast cells)
-attracts and triggers inflammatory cells (chemotaxis)
-enhances clearance of immune complexes
potential danger of the complement system and subsequent activation of the inflammatory response
anaphylactic shock
describe the cellular events that take place when the innate immune system responds to a cut on your foot
1) cut allows pathogens into body, causing resident effector cells to secrete cytokines
2) vasodilation and increased permeability allow fluid, protein, and inflammatory cells to leave blood and enter tissue
3) infected tissue becomes inflamed, causing redness, swelling, heat, and pain
how is a fever developed
macrophages produce cytokines: IL-6, TNF alpha, and IL-1B
which cytokines play a role in fever development?
IL-6
IL-1B
TNF alpha
besides fever, where do IL-6, IL-1B, and TNF alpha act in the body and what do they do?
liver: acute phase proteins (C reactive protein, mannose-binding lectin) activate complement
bone marrow and endothelium: neutrophil mobilization for phagocytosis of pathogens
hypothalamus: increased body temperature
fat and muscle: protein and energy mobilization to increase body temperature
the innate immune system provides _________, is __________, and is _______
-initial protection
-rapid
-always present
cell types involved in innate immune system
phagocytic cells (neutrophils, macrophages) and NK cells
broad components of the innate immune system include:
-complement system
-cytokines
T/F; inflammation is a normal and healthy response.
TRUE
innate immune system triggers:
adaptive immune response
adaptive immune response develops _______
slowly
is the innate immune response or adaptive immune response more effective?
adaptive
what triggers the adaptive immune response?
pathogens
the adaptive immune response is (highly/not very) specific
highly specific
Which statement is NOT TRUE about the innate immune system?
A) It induces inflammatory response by recruiting inflammatory cells to the site of infection.
B) Each time the body is exposed to an infectious agent, the immune response remains the same.
C) Specificity and immunological memory are involved.
D) The immune response occurs rapidly, usually within several hours.
C) Specificity and immunological memory are involved
memory and specificity are characteristics of the adaptive immune system.
Which two cell types are the most important phagocytic cells in the innate immune system?
-neutrophils
-macrophages
__________ proteins are a group of 30 proteins sequentially activated during infection, which promotes inflammation and pathogen destruction by phagocytosis.
complement
Which of the following is NOT an example of innate immunity?
a) inflammation
b) phagocytosis of pathogens by neutrophils
c) fever
d) antibody production
d) antibody production
T/F; Mast cell is the most important cell type in the release of histamine to promote inflammation.
TRUE
how do cytokines regulate inflammation
-dilation
-increased permeability
-recruit other immune cells
-activate NK cells
-associated w/ s/sx of inflammation and innate immunity
IFN-alpha and beta are mostly _______(bacterial/viral)__________
viral
what is the very first immune response to infection or injury?
inflammatory response
which cells are tissue resident cells (effector cells)
-macrophages
-mast cells
-dendritic cells
what causes macrophages to produce cytokines?
pathogens
what causes a systemic infection and what is the result?
macrophages release TNF-alpha into the bloodstream instead of the tissue where they normally would. This causes edema, decreased blood volume, and other complications which lead to death.
role of the nervous system in infection
enhances immune response
role of RBCs in immunity
can bind to DNA from pathogens to alert immune system to presence of pathogens and circulating cell-free mitochondrial DNA.
However, RBCs that carry snippets of DNA are killed, which can cause anemia