Lymphocyte Development and Activation
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
101 Terms
effector cells, memory cells
Once mature T cells are exported to the periphery, they can undergo antigen-induced activation and differentiation into — and —
At the early stages of T cell development, precursors do not express CD4 or CD8.
What is a double negative T cell?
Double negative thymocytes commit to the T cell lineage and begin to rearrange their TCR gene loci.
What happens during stages DN1-DN4?
DN thymocyte successfully rearranges its TCR-B chain and combines it with a surrogate pre-Ta chain
Cell proliferates and expresses both CD4 and CD8
DP now start rearranging their TCRa chain to form a complete TCRaB receptor, which is necessary for antigen recognition.
What characterizes the transition from double negative to double positive thymocytes?
Positive Selection: DP thymocytes must bind to self-MHC molecules with moderate affinity to survive. If they fail to recognize self-MHC, they undergo apoptosis (cell death).
Negative Selection: DP thymocytes that bind too strongly to self-antigens presented by MHC undergo apoptosis to prevent autoimmune responses.
Process of selection of double positive thymocytes
This depends on which MHC class they recognize.
CD4+ SP T cells recognize MHC Class II (helper T cells).
CD8+ SP T cells recognize MHC Class I (cytotoxic T cells).
What determines the lineage double positive thymocytes commit to after surviving selection
TCRγ(delta) T cells, (more innate-like in behavior),
NKT cells (recognize lipid antigens rather than MHC),
regulatory T cells
Most thymocytes develop into TCRαβ CD4+ or CD8+ T cells, but some DN and DP thymocytes develop into
Immature DP thymocytes interact with epithelial cells in the cortex of thymus to select DP thymocytes that can bind to self MHC.
If they cannot bind, the cells will die by apoptosis in 3-4 days.
A protective signal is provided when DP thymocytes bound to epithelial cells, preventing them from undergoing cell death.
DP thymocytes mature into SP (CD4+ or CD8+) thymocytes.
Process of positive selection
SP thymocytes (CD4+ or CD8+) interact with bone-marrow derived APCs (DC and macrophages) in the thymic medulla.
SP thymocytes bearing high-affinity receptors for self antigen/MHC (self-reactive cells) are eliminated.
Process of negative selection
bone marrow and thymus
Primary lymphoid organs
lymph nodes, spleen, gut-associated lymphoid tissue
Secondary lymphoid organs
2:1
normal ratio of helper to cytotoxic T cells
regulatory T cells (Treg)
CD4+CD25+ are mostly —, usually about 5-10% of T cells.
aB (only 5% have y-delta)
95% or T cells have this T cell receptor
disulfide
The two TCR chains are connected by a — bond
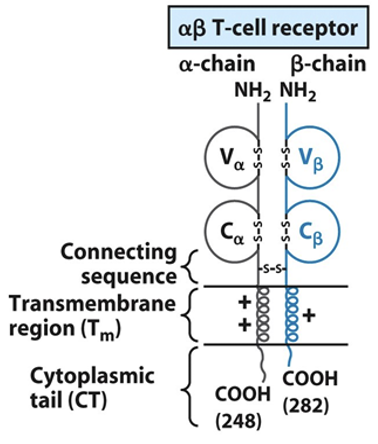
Receptor portion—Va and Vb are variable, connecting sequences are constant.
Transmembrane domain and short intracytoplasmic tails are present.
General structure of TCR
four TCR multigene families, each encoding one of the receptor chains (α, β, γ, and d).
Germline DNA contains these TCR gene families
V and J segments in the α-and γ-chain genes
and of
V, D, and J segments in the β- and d-chain genes.
Functional TCR genes are produced by rearrangements of — and —
RAG1/2 recombinase
T cells express — enzyme to catalyze V-J and V-D-J joining during TCR-gene rearrangement.
Vα-Jα
The α-chain DNA undergoes a variable-region — joining.
Dβ to Jβ first and then Vβ to DβJβ
The β-chain DNA undergoes two variable-region joinings:
TCR associates with CD3, forming the TCR-CD3 membrane complex.
CD3 is required for the surface expression of the TCR. Also, the CD3 is required for the initiation of the transduction signals as TCR has a very short intracellular tail that cannot signal.
Role of CD3 in TCR
immunoreceptor tyrosine-based activation motif (ITAM)
The intracellular domains of CD3 contain —, important for signal transduction.
CD4 is a monomeric membrane glycoprotein with 4 extracellular Ig-like domains, a transmembrane domain, and a long cytoplasmic tail containing serine (which can be phosphorylated).
The extracellular domains of CD4 bind to the β2 of MHC class II.
Role of CD4 in TCR
CD8 is a disulfide-linked heterodimer αβ. The chains contain a single extracellular domain, a transmembrane domain, and a cytoplasmic tail, containing several phosphorylation sites.
The extracellular domains of CD8 bind to the α3 of MHC class I.
Role of CD8 in TCR
TCR binds to MHC-peptide, engaging CD4 or CD8.
LCK phosphorylates ITAMs on the CD3 and ζ chains.
ZAP-70 binds to phosphorylated ITAMs and is activated.
Downstream signaling pathways are triggered, leading to T-cell activation, proliferation, and differentiation.
Summary of intracellular TCR signaling
CD28 on Th/Tc cells interacts with B7 (CD80/86) on antigen presenting cells. This interaction provides a crucial second signal (costimulatory signal) that ensures full activation of the T cell.
Purpose of CD28 on T cells
Interacts with LFA-3 on APC
and
Strengthens adhesion between T cell and APC
Purpose of CD2 on T cell
Interacts with ICAM-1 on APC,
Further stabilizes the interaction, ensuring prolonged T-cell activation
Purpose of LFA-1 on T cell
Substance that can be recognized by the antibody or by the TCR (T cells) when associated with MHC molecules.
What is an antigen?
Immunogenicity: The ability of a substance to induce an immune response (i.e., trigger the production of antibodies and/or activate T cells).
Antigenicity: The ability of a substance to bind specifically to an antibody or a T-cell receptor (TCR) when presented with MHC molecules.
All immunogens are antigens (because they can bind to immune receptors and also induce an immune response).
Not all antigens are immunogenic (some can bind to immune receptors but may not trigger a full immune response).
Immunogenicity vs. Antigenicity
small molecules that can bind to Abs but cannot by themselves induce an immune response (antigenic but not immunogenic). By conjugating to a large carrier protein, hapten (hapten-carrier complex) is immunogenic and elicits production of anti-hapten Abs.
What are haptans?
drugs, peptide hormones, and steroid hormones
Examples of haptans
Penicillin acts as a hapten, meaning it is too small to trigger an immune response on its own.
It covalently binds to RBC membrane proteins, modifying the RBC surface.
The immune system recognizes the modified RBCs as foreign and produces IgG antibodies against them.
These IgG-coated RBCs are then targeted for destruction by macrophages in the spleen and liver, leading to extravascular hemolysis.
Explain penicillin-induced hemolytic anemia
epitopes
Immune cells do not interact or recognize an entire immunogen molecule. Instead, they recognize discrete sites on the macromolecule called —
antigenic determinant
Epitope is also called —
Anything that induces cell proliferation unrelated to antigen-induced proliferation
Example: Lipopolysaccharide (LPS) on Gram (-) bacteria cell wall stimulates dendritic cell and B cell proliferation.
What is a mitogen?
Superantigens, such as bacterial exotoxins, can bypass the normal TCR-peptide-MHC recognition process by binding directly to the outer part of the TCR and the MHC class II molecule on the APC. Superantigens don’t require a specific peptide to be presented in the MHC groove. Instead, they bind to a region of the TCR away from the normal peptide-binding site, and this results in non-specific activation of a large number of T cells. This causes the T cells to become activated regardless of their specificity.
How superantigens work
Cross-reactivity is the ability of an antibody or TCR to bind to different antigens with similar or identical epitopes. This phenomenon can lead to broader immune recognition or, in some cases, harmful autoimmune reactions if the immune system mistakenly attacks the body's own tissues due to similarities in molecular structures.
What is cross-reactivity?
B cells express membrane bound immunoglobulins (IgM) on their surface. Binding of IgM to antigen activates the B cell. B cell proliferates, creating clones that all carry the same IgM. Some differentiate into plasma cells and secrete antibodies into the bloodstream. Antibodies bind their antigen, which helps neutralize pathogen and mark it for destruction by other immune components like phagocytes.
Process of antibodies being expressed and then neutralizing pathogen.
two identical heavy (H) chains and two identical light (L) chains
Abs are heterodimers composed of —
Both H chains and L chains consist of amino-terminal variable (V) regions and carboxyl-terminal constant (C) regions.
V regions participate in Ag recognition.
C regions of H chains mediate effector function
V and C regions of Ab: location and function
disulfide
Antibodies are stabilized by — bonds
The hinge region is a flexible segment of the antibody (Ab) structure that lies between the Fab (antigen-binding) regions and the Fc (constant) region of the antibody. It plays a crucial role in allowing the antibody to adapt and maintain its functional shape when interacting with antigens.
Hinge region:
heavy chains
This determines an antibody’s class
hinge
IgE and IgM lack a — region
mIgM
Ig expressed by immature B cells
both mIgM and mIgD
Ig expressed by mature B cells
Memory B cells typically express only one antibody class (mIgM, mIgG, mIgA, or mIgE) depending on the immune response and class switching that occurred after their activation
Ig expressed by memory B cells
IgG
Most abundant Ig in serum
placenta (only class that does so)
IgG crosses the —
classic
IgG activates complement via the — pathway
FcRn (or called FcRB)
— Receptor Transports IgG from the Bloodstream into Tissues
FcRn
Maternal IgG is transported by the — receptor across the placenta to the fetus.
Membrane-bound IgM (mIgM): On the surface of B cells, IgM is expressed as a monomer. A monomer is a single Ig molecule with two heavy chains and two light chains, which is the form that functions as the B cell receptor (BCR).
Secreted IgM: Once B cells are activated, they can secrete IgM as a pentamer (five IgM monomers joined together). This form is more effective in certain immune functions than the monomeric form found on B cells.
Structure of bound vs secreted IgM
J chain, disulfide
Each IgM pentamer is held together by a —, a small polypeptide, which is attached through — bonds
10
The pentameric form of IgM contains — antigen-binding sites in total
large (The larger structure allows it to capture and neutralize antigens more efficiently.)
IgM is particularly effective at binding to — particles, such as viruses, bacteria, or cells, due to its pentameric structure and the 10 available binding sites.
IgM
Which is the most efficient antibody in activating the complement system via the classical pathway?
Binds to the Fc region of antibodies (IgG) that have opsonized pathogens, leading to phagocytosis and destruction of the pathogen.
Function of Fc Receptor on MΦ (macrophages):
Binds to C3b, a complement protein, which is deposited on pathogens during the complement activation process, and this also leads to phagocytosis and pathogen elimination.
Function of CR1 Receptor on MΦ (macrophages):
monomer in serum, dimer in secretions
10-15% of serum Igs
Major class of Ig in secretions like saliva, tears, and mucus; breast milk provides IgA for newborns (passive immunity)
IgA: structure, prevalence, sources
The SC (secretory component) produced by epithelial cells.
This protects IgA from degradation in secretions
IgE, monomer
Involved in allergic reactions, rare because it binds tightly to Fc receptors on basophils and mast cells even before interacting with antigen
Eosinophils have Fc receptors for IgE and binding of eosinophils to IgE-coated helminths results in killing of the parasite.
Least common Ig and its structure and function
When IgE antibodies are produced in response to an allergen, they bind to the Fc receptors on the surface of mast cells and basophils. This binding "sensitizes" these cells, meaning they are now primed to respond if they encounter the same allergen again.
The next time the person is exposed to the same allergen, the allergen molecules can bind to multiple IgE antibodies that are already attached to the Fc receptors on the mast cells or basophils.Upon activation by the crosslinking of IgE, the mast cells and basophils undergo degranulation, releasing histamine and other mediators.
Explain process of IgE-mediated allergy
Neutralization of pathogens
Opsonization of Ags to promote phagocytosis
Activation of complement to enhance opsonization
Three main ways Abs participate in host defense
the strength of interaction between an epitope and an antibody’s antigen binding site (single binding event). The higher the affinity of the antibody for the antigen, the more stable will be the interaction.
Antibody affinity measures —
the overall binding strength of an antibody-antigen complex (accounting for all binding interactions)
Antibody avidity measures —
valency (number of antigen binding sites)
-all antibodies are multivalent
The greater an immunoglobulin’s —, the greater the amount of antigen it can bind.
mIg is the form of the antibody that is membrane-bound on B cells. It serves as the B cell receptor (BCR). The cytoplasmic tail of mIg is very short. This means that the mIg itself cannot directly transmit any signals to the inside of the B cell when it binds to an antigen.
To overcome this, mIg associates with Igα (CD79a) and Igβ (CD79b), which are transmembrane proteins. These proteins have longer cytoplasmic tails that are capable of transmitting signals inside the cell.
When the mIg binds to an antigen, this causes a conformational change in the BCR complex. The Igα/Igβ heterodimers transmit this change inside the B cell through their long cytoplasmic tails.
How BCRs signal intracellularly
CD19 is a cell surface molecule that is expressed on B cells.
It has a long cytoplasmic tail, which is important for signal transduction. The long tail allows CD19 to transmit signals inside the cell when it is phosphorylated.
CD19 serves as a critical co-receptor in the B cell activation process, acting as a signal amplifier.
CD19 function
CR2 (also known as CD21) is a receptor on B cells that specifically binds to C3d, which is a fragment of the complement protein C3.
C3d is deposited on the surface of an antigen (Ag) during the complement cascade when the immune system marks pathogens for destruction.
CR2 acts as a receptor for C3d (C3d is the specific ligand for CR2), and it helps enhance the immune response by facilitating B cell activation when the BCR binds to the antigen.
CR2/CD21 function
TAPA-1, also called CD81, is another surface protein that is involved in organizing the co-receptor complex.
It helps maintain the structure and function of the CD19-CR2 complex on the B cell surface, and it is involved in signal transduction when the complex interacts with antigens.
TAPA-1/CD81 function
tri-molecular complex (C3d/Ag/BCR)
The B cell receptor (BCR), which is made up of a membrane-bound immunoglobulin (Ig), recognizes and binds to a specific antigen (Ag).
The binding of C3d to CR2 on the B cell surface, in conjunction with the binding of the BCR to the antigen, forms a —.
V(D)J rearrangement
— brings together multiple germline gene segments that may combine randomly, and different combinations produce different antigen receptors that contributes to the diversity of TCR and BCR.
B1: fetal liver
B2: after birth in bone marrow
B1 vs B2 cells: when/where they are first produced
B1: peritoneal and pleural cavities
B2: secondary lymphoid organs, blood
B1 vs B2 cells: primary location
B1: self-renewing
B2: replaced from bone marrow
B1 vs B2 cells: mode of renewal
B1: first line of defense, innate-like immunity
B2: adaptive immune responses, antigen-specific immunity
B1 vs B2 cells: function
B1: limited
B2: strong memory and long-term immunity
B1 vs B2 cells: memory
encounter antigen, become activated, undergo clonal expansion, and differentiate into effector cells.
Secondary lymphoid organs are where lymphocytes —
Cortex (B cell zone)
Paracortex (T cell zone)
Medulla (plasma cells)
Three regions of lymph node
antigens
Lymph nodes trap — from local tissues
Contains B cells, macrophages, and follicular dendritic cells (DCs) arranged in primary follicles.
Morphology of cortex
After antigenic challenge, the primary follicles enlarge into secondary follicles, each containing a germinal center.
What happens to lymph node cortex after antigenic challenge
T cells, dendritic cells
The paracortex is populated largely by — and contains interdigitating —
MHC II molecules
In the paracortex, DCs express high levels of — to present Ags to activate Th cells
plasma cells secreting antibodies
In the medulla, there are few lymphocyte, mostly —
spleen
First line of defense against blood borne pathogens
marginal zone
In the spleen, a specialized region of macrophages and B cells known as the — borders the white pulp.
Contains macrophages and red blood cells.
This is the place where old and defective red blood cells are destroyed and removed.
Red pulp contents and function
periarterial lymphoid sheath (PALS) which contains mostly T cells (T-cell zone)
White pulp surrounds the arteries forming the —
B cells organized in primary follicles
The marginal zone of the spleen is rich in —
Intestinal epithelium is referred to as gut-associated lymphoid tissue (GALT).
Cutaneous epithelium: Cutaneous-associated lymphoid tissue
MALT in intestines and cutaneous tissues is called:
lamina propria
In GALT, the — contains B cells, plasma cells, activated Th cells, and macrophages in loose clusters, forming lymphoid follicles.
Peyer's patches
In GALT, the submucosa contains — that nodule with secondary follicles with germinal centers.
CD8+ T cells with a γd T cell receptor type
The outer mucosal epithelial layer contains intraepithelial lymphocytes (IELs); the majority are —.
cytokines
The outer epidermal layer of skin contains keratinocytes (epithelial cells) that can secrete —.
skin DCs; After internalizing Ag by phagocytosis or endocytosis, Langerhans cells migrate from skin to the lymph nodes to activate naïve CD4+ T cells.
Langerhans cells function: