Chemistry 6.1 - Bonding Between Metals and Nonmetals
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What happens when a metal reacts with a nonmetal?
Each metal atom loses electrons to form a cation with a full outer shell,
and each nonmetal atom gains electrons (from the metal) to form an anion with a full outer shell.
How do both nonmetals and metals when they react obey the Octet Rule?
The Octet Rule asserts that atoms will lose or gain electrons to form an ion with a full outer shell when they react with other elements to form compounds.
How do metal atoms and nonmetal atoms react to form ionic compounds? (using info from the periodic table)
Metal atoms lose electrons to achieve the same electron configuration as a noble gas, while nonmetal atoms gain electrons to do the same.
What happens when sodium reacts with chlorine?
Each Sodium atom loses one electron to form a sodium cation (Na+), and each chlorine atom gains one electron to form a chloride anion (Cl-)
What is ionic bonding?
The electrostatic attraction between oppositely charged ions in the giant ionic lattice.
What are ionic compounds?
Compounds with a giant ionic lattice structure.
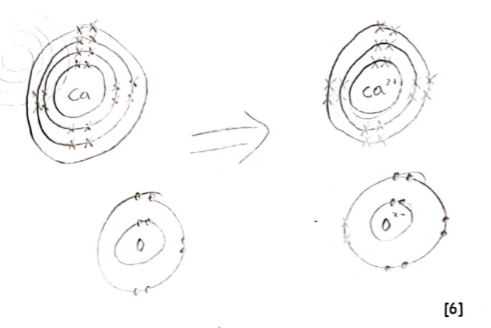
Burning calcium in oxygen produces a white powder called calcium oxide.
Use a diagram to explain, using full electronic structures, how calcium and oxygen bond to form calcium oxide.
Here is the diagram you should draw
Silicon dioxide is heated with sodium carbonate and magnesium oxide to make glass. Sodium carbonate and magnesium oxide contain ionic bonding.
Explain, using full electronic structures, how atoms of magnesium and atoms of oxygen form ions and bond to produce magnesium oxide.
The electronic configuration of magnesium is 2, 8, 2.
The electronic configuration of oxygen is 2, 6.
Each magnesium atom loses two electrons to form an Mg2+ ion.
Each oxygen atom gains two electrons to form a O2- ion.
The electronic configuration of an Mg2+ ion is 2, 8.
The electronic configuration of an O2- ion is 2, 8.
The Mg2+ ions and the O2- ions are held together by the electrostatic attraction between oppositely charged ions in the lattice.
(((((Any 6)))))
How is the formula for aluminium oxide determined?
Aluminium reacts with oxygen to form Al3+ and O2-. Giving the formula Al2O3. 2 Al3+ = 6 electrons & 3 O2- = 6 electrons.
What is the formula for magnesium fluoride?
The compound contains (Mg2+) ions and fluoride (F-) ions
One Mg2+ ion is formed for every 2 F- ions formed
The formula is MgF2
What is the formula for sodium oxide?
The compound contains Sodium (Na+) ions and oxide (O2-) ions
2 Na+ ions are formed for every O2- ion formed
The formula is Na2O
What is the formula for zinc chloride?
The compound contains zinc (Zn2+) ions and chloride (Cl-) ions
1 Zn2+ ion is formed for every 2 Cl- ions formed
The formula is ZnCl2
What is the formula for iron(II) sulfide.
The compound contains iron(II) (Fe2+) ions and sulfide (S2-) ions
1 Fe2+ ion is formed for every S2- ion formed
The formula is FeS
What are the ions in Aluminium Bromide? Please also write the formula for each compound.
Ions in Formula: 1 Al3+ and 3Br-
Formula: AlBr3
What are the ions in Copper(II) chloride? Please also write the formula for each compound.
Ions in Formula: 1Cu2+ and 2Cl-
Formula: CuCl2
What are the ions in Zinc sulfide? Please also write the formula for each compound.
Ions in Formula: 1Zn2+ and 1S2-
Formula: ZnS
Name the compound with the formula BaCl2. Please also list the atoms in the formula of each compound.
Name: Barium Chloride
Atoms in Formula: 1Ba and 2Cl
Name the compound with the formula Mg3N2. Please also list the atoms in the formula of each compound.
Name: Magnesium Nitrate
Atoms in Formula: 3Mg and 2N