Ch2- Atomic and Electronic Structure
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
what does the atomic # signify
number of protons
number of the box the element is in on the periodic table
atomic # never changes- T or F?
TRUE
proton number never changes- T or F?
true
what is the superscript? what is the subscript?
superscript= mass of element (atomic weight)
subscript= atomic # of element and # of protons
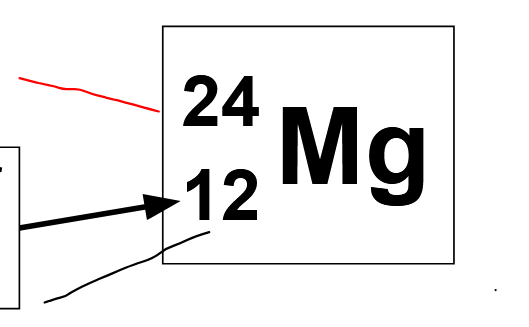
red= atomic weight/ molar mass
black= atomic #/ # of protons
equation for atomic mass (superscript)- 2 possible
# of protons + # of neutrons= atomic weight/ molar mass of element
or atomic # + # of neutrons= atomic weight/ molar mass

where are the protons and neutrons kept in an atom?
in the nucleus
mass number can change- T or F? and what changes?
TRUE- mass number can change, the number of neutrons change
what are isotopes?
atoms of the same element that have different mass (due to differing amounts of neutrons, but protons are ALWAYS THE SAME)
what is quantization?
when a position can only exist at certain distinct levels, but NOT in-between those levels (ex. you can stand on steps of the stairs, but not in between those steps, therefore your position on a set of stairs is quantized)
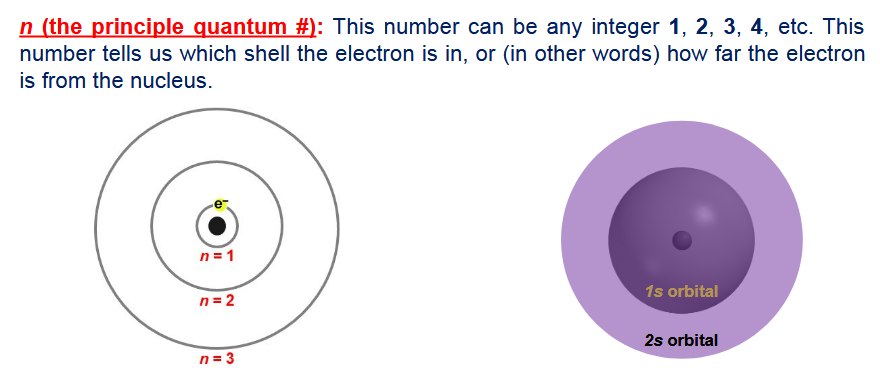
what is quantized in an atom?
electron’s distances from their nuclei are quantized (there is no n=1/2 shell
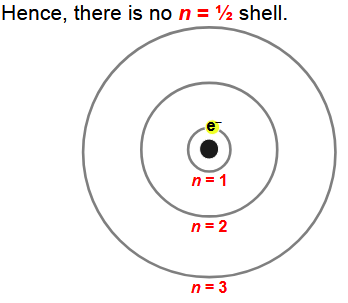
how does the distance between each energy shell starting from the nucleus of an atom change
the distance between each energy shell starts big and then eventually gets smaller as you get further away from the nucleus
how do electrons travel around the nucleus?
in three-dimensional regions of space called orbitals (also known as atomic orbitals for single atoms, or molecular orbitals for molecules)
4 different kinds of orbitals
s, p, d, f- orbitals
what are orbitals exactly?
regions of space around an atom’s nucleus, where its electrons “live”- they are like empty parking places for electrons
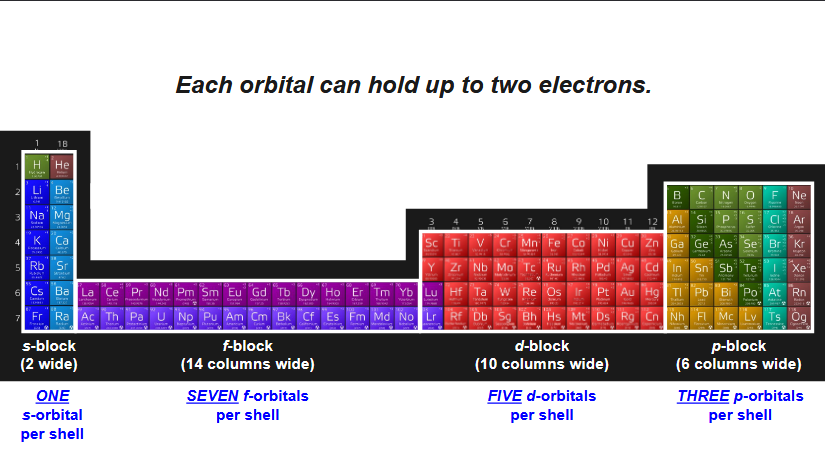
how many s-orbitals per shell? how many electrons are housed by s-orbitals? which portion of periodic table uses s-orbitals?
1 s-orbital per shell, 2 electrons max in each each orbital, first two columns of periodic table

more details on s-orbitals
shaped like 3Dspheres
1s orbital surrounds the nucleus at an n=1 distance
2s orbital surrounds the nucleus and 1s orbital at an n=2 distance etc
lower energy orbitals (lower-n) and their electrons are buried inside higher-energy orbitals (higher-n) like russian nesting dolls (same for p, d, f orbitals)

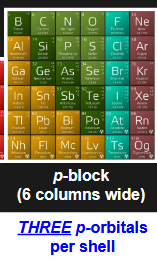
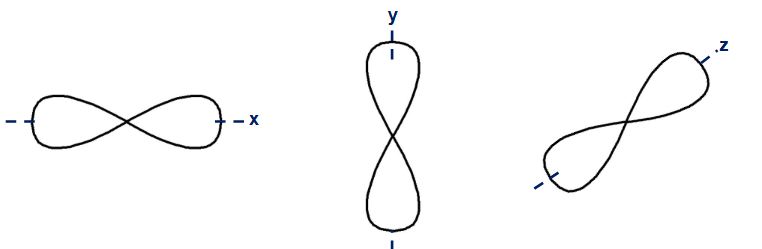
how many p-orbitals per shell? how many electrons are housed by p-orbitals? which portion of periodic table uses p-orbitals?
2p shell
3 p- orbitals per shell (x-axis, y-axis, z-axis)
6 electrons max (2 electrons for each orbital)
right side of periodic table
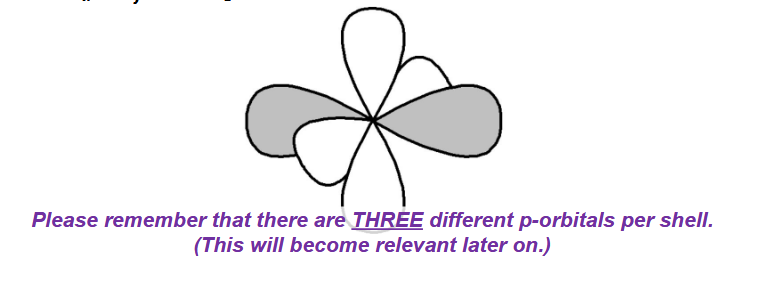
how are p-orbitals shaped?
like dumbells
how many d-orbitals per shell? how many electrons are housed by d-orbitals? which portion of periodic table uses d-orbitals? how are they shaped
5 d-orbitals per shell
10 electrons max
d-block= 10 columns wide (transition metals, the block inbetween left (s) and right (p)
first 4 look like four-leaf clovers, last one has donut/dumbell shape
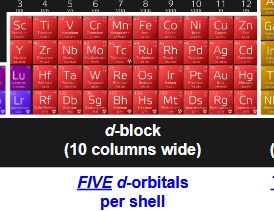
how does the funky way that d-orbitals are shaped affect the appearance of transition metals, and why?
makes transition metals brightly-colored
the funky shape allows electrons in d-orbitals to absorb colored light and be promoted to higher energy orbitals
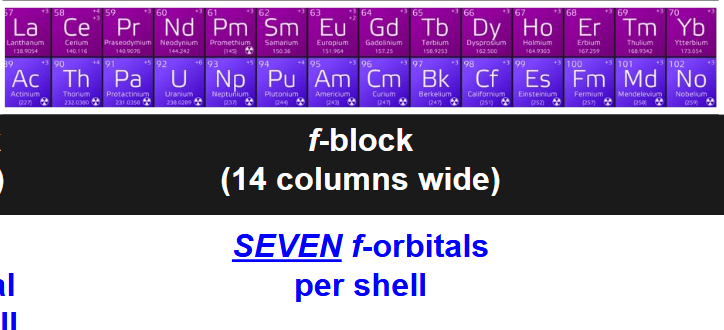
how many f-orbitals per shell? how many electrons are housed by f-orbitals? which portion of periodic table uses f-orbitals? how are they shaped?
7 f-orbitals per shell
14 electrons housed by f-orbitals
last 2 rows bottom of periodic table (in betwen s and d-block originally)
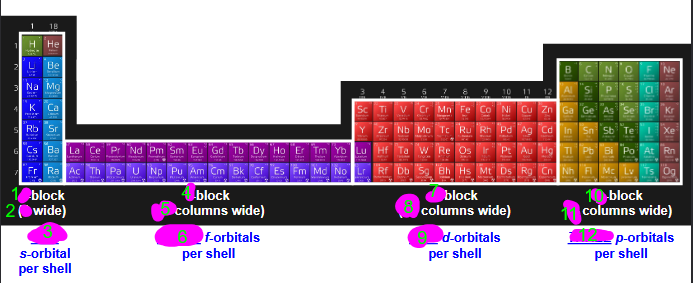
fill this out
what is the numberical system that scientists use for describing an electron’s location, or address?
quantum numbershow m
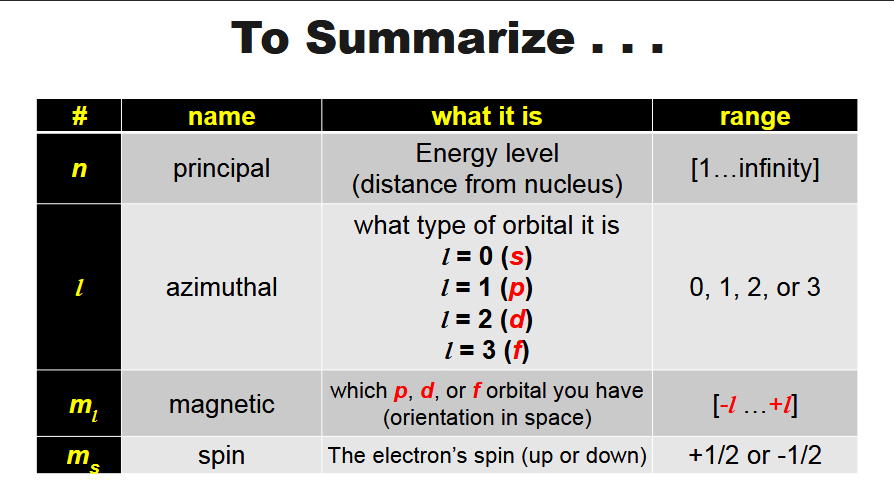
how many quantum numbers are there? and what are they
4, n, l, ml, ms
what does n (quantum number) stand for?
principle quantum number- can be any integer from 1- infinity, tells us which shell the electron is in (or how far the electron is from the nucleus)
what is l (quantum number)
the azimuthal quantum #- it represents which orbital the electron belongs in (s, p, d, or f, which correspond to numbers 0,1,2,3

what is mℓ (quantum number)? what does it represent?
it represents which one of the p, d, f -orbitals it’s in ex. p-orbital is -1, 0, 1

what is ms? what does it represent?
last quantum number, it could be either - or + ½
for any of the two electrons occupying the same orbital, one is assigned to a +1/2 spin and the other a -1/2 spin
memorize

practice problem
answer= D

practice problem
a. 0, 1, 2, 4
b. -2, -1, 0, 1, 2
c. 2, 3

practice problem
answer= a
what is an electron configuration?
it is a list of all the electrons in the atom and the orbitals that they occupy
what is the electron configuration of oxygen?
1s²2s²2p^4
how can you add up all the electrons in the configuration of the element to check your answer?
adding up all the superscripts adds up all the electrons in the atoms, which = the atomic number of element (also # of protons since # of protons = # of electrons
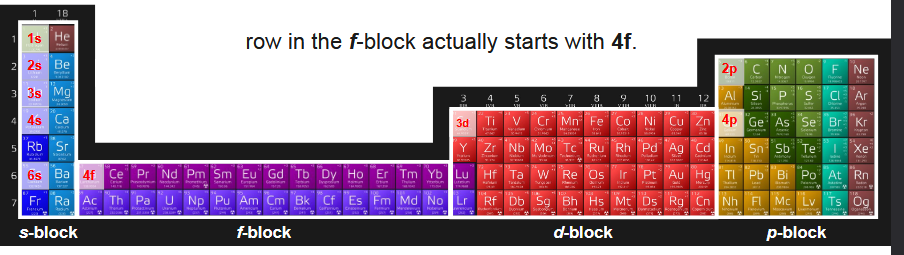
what are the exceptions in the numbering of the rows in the periodic table?
the d block first row is 3s, when it is on the 4s row (drop 1, or 4-1=3)
the f block first row is 4s, when it is on the 6s row (fall 2, or 6-2=4)
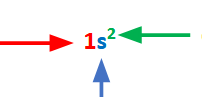
blue= ?
red=?
green=?
red= principle quantum number= n
blue= the orbital the particular electrons are in, in this case, s-orbital, and l=0
green= represents how many electrons there are in this orbital in question
what is hund’s rule?
electrons fill up these orbitals one at a time (electrons won’t pair up to occupy the same orbital until there’s no option)
how do you know if elements have similar properties?
if they are in the same group (meaning column), like Si is similar to C
how do you write an electron’s configuration in a “condensed” way?
write the elemental symbol of the noble gas that comes just before the element, and then the configuration that comes after that
noble gases are the last column
ex. what is the condensed configuration of Br?
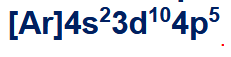

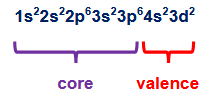
what are valence and core electrons? how do you count how many valence electrons an element has? how many valence electrons does this atom have?
valence electrons are the electrons in the outermost shells, core electrons are the ones that are buried inside the inner shells
find the higher order shells (n), and add the superscripts
ex. 7 valence electrons
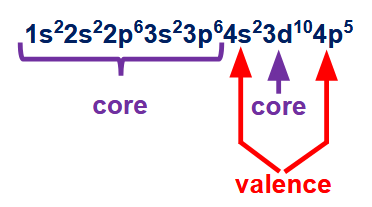
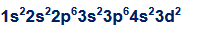
what is one exception about valence electrons? how many valence electrons are in this configuration?
d-orbital electrons will always be valence electrons
5 valence electrons

the larger an electron’s principle quantum number (n), the… (3 things)
the higher the distance from the nucleus
higher the energy
the higher the reactivity

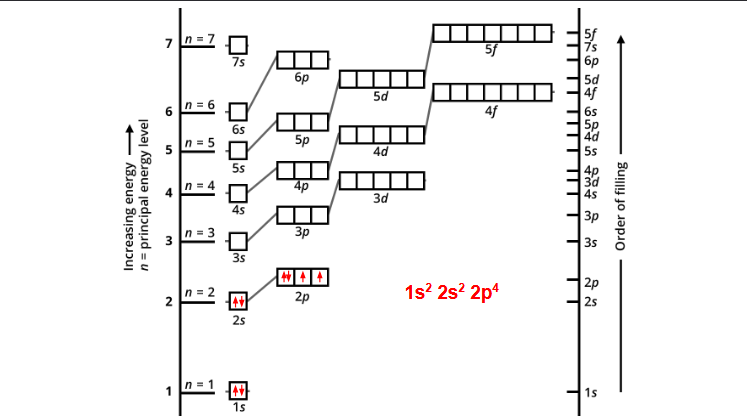
how do you fill this up?
1st box, you do you arrow up, one down
3rd box, do all arrows up first, across the row, then one arrow down last
Aufbau principle- ?
Hund’s rule-?
pauli exclusion principle- ?
aufbau principle= electrons fill the lowest energy orbitals first (bottom first)
hund’s rule= don’t pair up electrons until you have to
pauli exclusion principle= no two electrons in the same atom can have the same four quantum numbers (CAN HAVE THE SAME FIRST 3 QUANTUM NUMBERS, BUT NOT THE LAST ONE)

which elements are the exceptions to the electron configuration rule?
[Cr] [Mo] [Cu] [Ag] [Au]
chromium, molybdenum, copper, silver, gold
for each of them you add an electron from the s-orbital to the d-orbital
what would the electron configuration for Cr and Mo look like? how many advantages are there to the exception configuration rule, and what are they?
3d⁵4s¹
2 advantages
shift electron from higher, more UNSTABLE, more REACTIVE energy level, to a more stable/ less reactive energy level (4s—>3d)
no more empty shell in 3d orbital, all shells filled with at least 1 electron
what would the electron configuration for Cu, Ag, Au look like? how many advantages are there to the exception configuration rule, and what are they?
4s¹ 3d¹⁰
1 advantage
move paired electron from a more UNSTABLE, and more REACTIVE energy level to a more stable/less reaction energy level (4s—>3d)
3d shell is at a higher energy level than the 4s when both have 0 electrons, but as you start adding electrons, 4s is at a higher energy level- T or F
True
empty shell is unstable- T or F
true
when there is an unpaired electron in the shell, or a full shell, it is more stable
explain excited electron configuration
when an atom absorbs a photon of light, or other form of energy, its electrons can get “promoted” up to a higher-energy shell or orbital (an electron will jump to a higher energy shell)
what should you keep in mind about excited electron configuration?
excited electrons can skip entire orbitals (4s—> 4p, entirely skipping the 3d orbital)
once an atom gets to its excited state, eventually its electron will relax and go back down to its ground state- T or F
TRUE
when an electron gets excited, explain how energy plays a role
it requires energy for the electron to jump orbitals and get excited
when an electron eventually relaxes back to its ground state, explain how energy plays a role
the electron gives off energy when it relaxes back to ground statew
what forms does the electron give off its energy when it relaxes from a higher order to state to a lower one?
it gives off its energy in the form of heat, light, or color

tip to solve this question faster
add electrons in configuration and find its atomic number

important detail to remember about these types of questions
for IONS, take away the electrons from the HIGHEST energy orbital first
the configuration for Co is [Ar] 4s² 3d⁷, so you take the electrons from 4s, to have 3 unpaired electrons

tip for this question
add up the electrons to find the atomic #
paramagnetic…
UNpara (unpaired), it has unpaired electrons, paramagnetic elements are ATTRACTED TO MAGNETS (think of the magnets filling the empty space of the unpaired electron shells
diamagnetic means
all electrons are paired
diamagnetic electrons are slightly repelled by magnetic fields
quick tip about paramagnetic elements
if elements have an odd number of electrons (or atomic #), they are PARAMAGNETIC
if even, they can be either dia or para
what does it mean when they ask which of the following would interact with a magnetic field?
means which ones are paramagnetic (unpaired electrons, odd number of electrons/atomic number, attracted to magnet)
what does a spectrophotometer do?
it uses a prism to split the colors (that each element emits) apart into their individual pieces
this creates a line emission spectrum, which is unique for each individual element (like a fingerprint)wh
what is a line emission spectrum
shows all the different lights a molecule emits, giving us a sort of fingerprint of the elements it consists of
energy of the photon is directly related to
frequency of a photon
energy of a photon is inversely related to
the wavelength
think of the equation for energy of a photon, frequency and wavelenth
energy = frequency = 1/wavelength

memorize

shorter wavelength (more compressed)
= higher energy, higher frequency
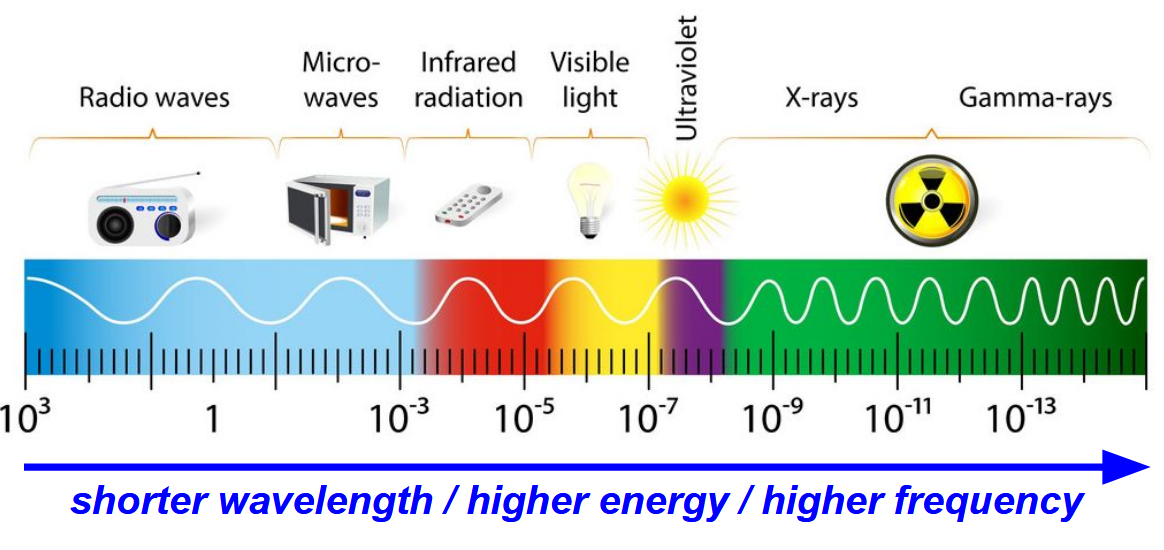
longer wavelengths (more elongated, sluggish)
lower energy, lower frequency
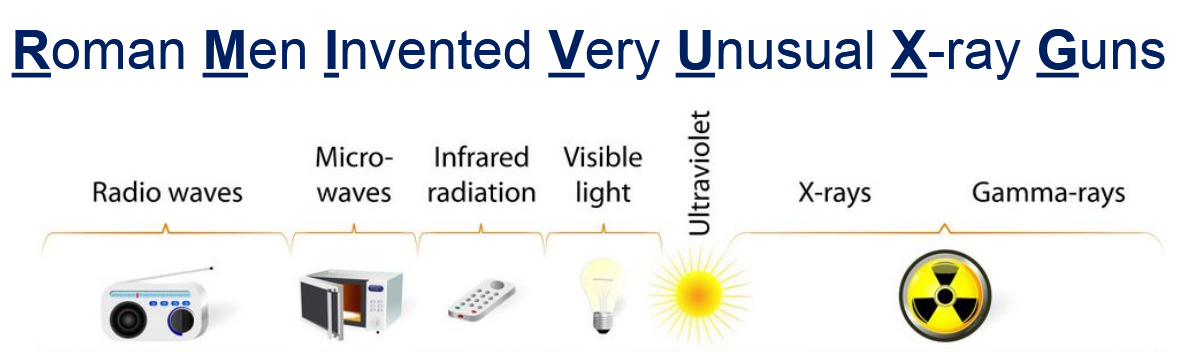
what is the mnemonic for remembering the different types of electromagnetic radiation
Roman Men Invented Very Unusual X-ray Guns