L2
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
What is the function of Pyruvate dehydrogenase?
converts pyruvate to acetyl-CoA,
producing CO₂ and NADH
What type of reaction does PDH catalyse?
oxidative decarboxylation reaction
Where does the PDH catalysing reaction occur?
mitochondria
Why is the PDH reaction metabolically important?
irreversible
commits pyruvate to energy production via the citric acid cycle
Why is pyruvate described as being at a central point in metabolism?
links glycolysis
to citric acid cycle and other metabolic cycles
What pathway does acetyl-CoA enter after PDH?
citric acid cycle
Is PDH a single enzyme or complex?
a large multi enzyme complex
between 5 and 10 ×106 Da
What are the 3 enzyme components of PDH?
E1 = Pyruvate dehydrogenase
E2 = Dihydrolipoyl transacetylase
E3 = Dihydrolipoyl dehydrogenase
Why does PDH require multiple enzymes for one reaction?
To allow coordinated catalysis
increase efficiency
prevent side reactions
How many cofactors are required for PDH activity?
5
many are derived from vitamins
Why do vitamin deficiencies impair PDH activity?
Because several of the PDH cofactors are derived from vitamins meaning there will be a reduced energy production when deficient
What is the role of lipoic acid in PDH?
It forms a flexible, movable arm
transfers intermediates between active sites
Which PDH subunit contains the lipoamide arm?
E2 (dihydrolipoyl transacetylase)
Why is the lipoamide arm important?
allows substrate channelling
increasing reaction speed and efficiency
What reaction does E1 catalyse?
the decarboxylation of pyruvate
hydroxyethyl-TPP

What happens to the hydroxyethyl group after decarboxylation?
hydroxyethyl group transferred to lipoamide
AND it is oxidised to an acetyl group
Two electrons left behind from decarboxylation transferred to E2
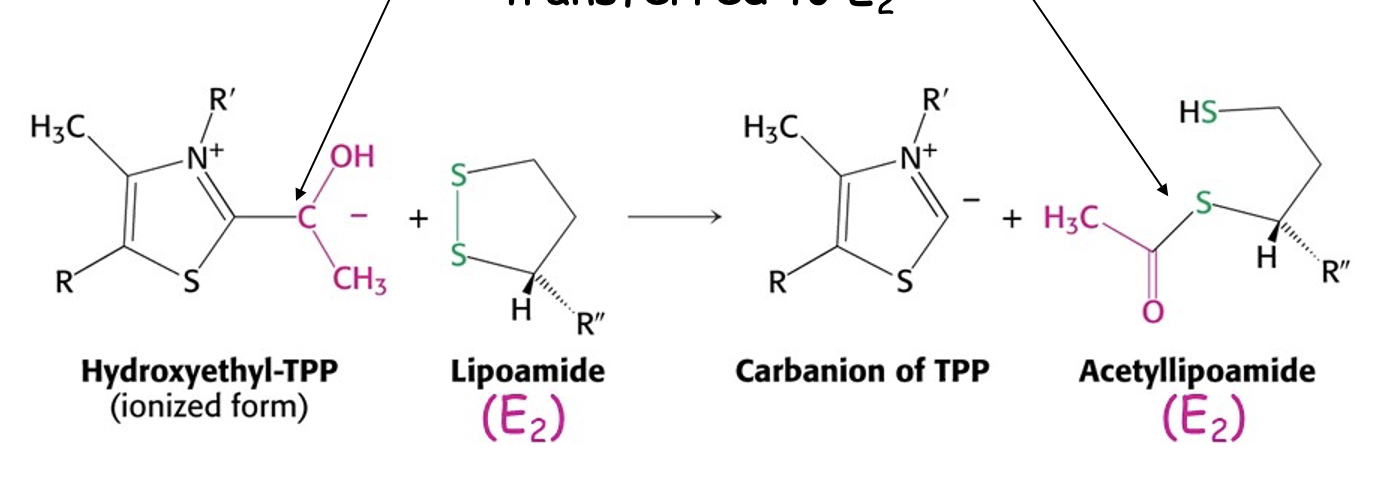
What is the role of E2 in PDH?
transfer of the acetyl group to a CoA
forming acetyl CoA
(joining them)
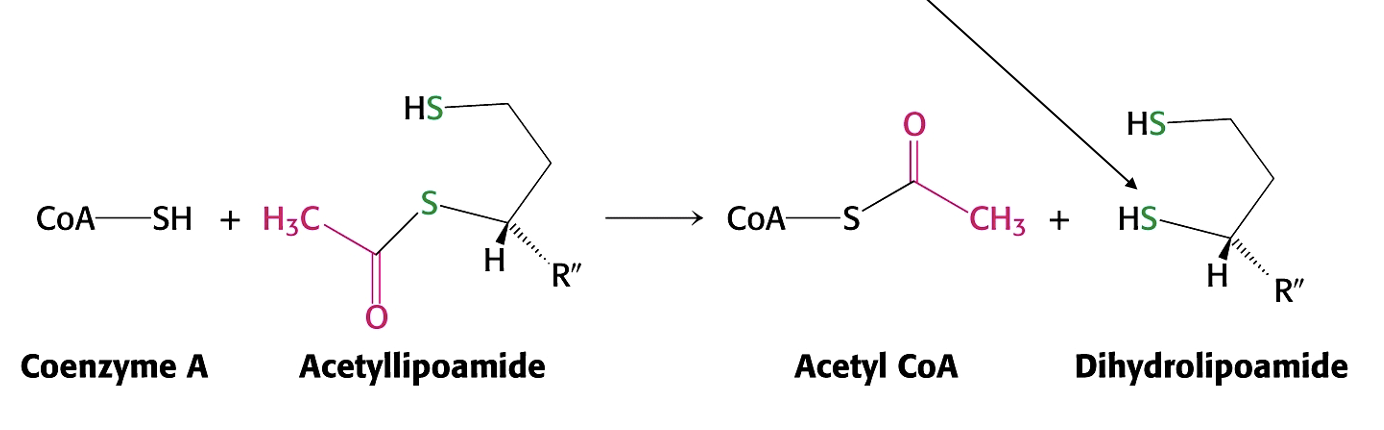
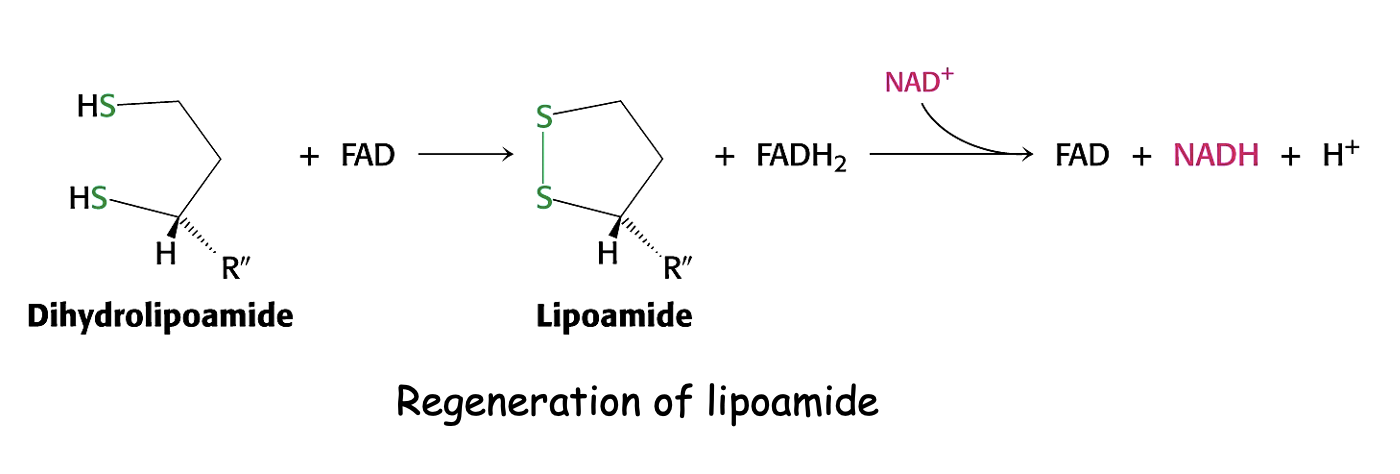
What is the role of E3 in PDH?
regeneration of oxidised lipoamide via FAD and NAD+
producing NADH - electron carrier
(reduction of FAD)
Electrons move from FADH2 to NAD to make NADH and FAD
Why are PDH intermediates not released into the solution?
They are highly bound
transfer between enzymes, reducing side reactions
What advantages does the PDH complex structure provide?
Maximised efficiency, speed, and control of the overall reaction
Why must PDH be regulated?
Because it catalyses an irreversible commitment to energy production
Which molecules inhibit PDH activity?
NADH
acetyl-CoA
ATP
(reaction products)
Which molecules activate PDH activity?
NAD⁺
ADP
AMP
CoA
(reactants)
How is PDH primarily regulated?
By phosphorylation and dephosphorylation of the E1 subunit.
What is the effect of PDH kinase activity?
Phosphorylation → PDH inactivation
What activates PDH kinase?
ATP
NADH
acetyl-CoA
What inhibits PDH kinase?
NAD⁺
ADP/AMP
CoA
What is the role of phosphatase?
Dephosphorylates E1
→ activates PDH
What stimulates PDH phosphatase in muscle?
Ca2+
especially during muscle contraction
What is beriberi and how does it relate to PDH?
thiamine deficiency- vitamin B₁
that impairs PDH
reducing ATP production
What metabolic changes are seen in PDH impairment?
Elevated pyruvate and lactate in the blood
How do mercury and arsenite poisoning affect metabolism?
They inhibit PDH
causing beriberi-like symptoms.
Why is pyruvate described as as a metabolic pathways?
it can be metabolised by five different pathways
depending on cellular conditions
What happens to pyruvate under anaerobic conditions in muscle?
It is reduced to lactate by lactate dehydrogenase
to regenerate NAD⁺
so glycolysis can continue
Why does lactate accumulation cause muscle cramping?
Lactate is a metabolic dead end in muscle
accumulates when oxygen is limited
How can pyruvate contribute to glucose or glycogen replenishment?
converted to oxaloacetate by pyruvate carboxylase
in glucose-depleted conditions
How is pyruvate linked to amino acid metabolism?
Pyruvate can be transaminated to alanine
reaction is reversible and depends on amino acid availability
What happens to pyruvate in microorganisms during fermentation?
It can be converted to ethanol
Which PDH subunit uses thiamine pyrophosphate (TPP)?
E1
Which vitamin is required to make TPP?
Vitamin B₁ -thiamine
Which PDH subunit uses Coenzyme A?
E2
Which vitamin is Coenzyme A derived from?
Vitamin B₅
Which PDH subunit contains lipoamide?
E2
Is lipoamide dietary or synthesised by the body?
Synthesised
→ non-dietary
Which PDH subunit uses FAD?
E3
Which vitamin is FAD derived from?
Vitamin B₂
Which PDH subunit interacts with NAD⁺?
E3
E3
Which vitamin is NAD⁺ derived from?
Vitamin B₃
Why is TPP essential for pyruvate decarboxylation?
It forms a reactive carbanion
that attacks the carbonyl carbon of pyruvate
What happens when the TPP carbanion reacts with pyruvate?
A carbon–carbon bond forms
triggering CO₂ release
What happens to the electrons left after decarboxylation?
They remain on the hydroxyethyl-TPP intermediate
Why is the hydroxyethyl-TPP intermediate unstable?
It is electron-rich
readily transfers electrons
What role does lipoamide play in PDH?
It accepts electrons and the acetyl group, then transfers them to other subunits
Why is lipoamide described as a “flexible arm”?
It is attached to E2 via a long (~14 Å) arm that reaches multiple active sites
Trace the path of electrons during the PDH reaction.
Pyruvate → TPP → lipoamide → FAD → NAD⁺ → electron transport chain
Why does thiamine deficiency cause lactic acidosis?
PDH is impaired, so pyruvate is shunted to lactate to regenerate NAD⁺
How do mercury and arsenite inhibit PDH?
They bind to lipoamide
rendering it inactive via chelation
What is PDH phosphatase stimulated by?
cytosolic Ca2+
important in muscles