general chemistry 8: the gas phase
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
what is the definition of:
theoretical gas whose molecules negligible space and whose collisions are perfectly elastic. the gases behave ideally under reasonably increase temperatures and decreases pressure
ideal gas
2
New cards
units of ideal gas
STP: 273 k (oc), 1 atm
1 mol gas: at STP 1 mol of gas = 22.4 L
units: a at = 760 mmhg = 760 torr = 101.3 kpa = 14.7 psi
3
New cards
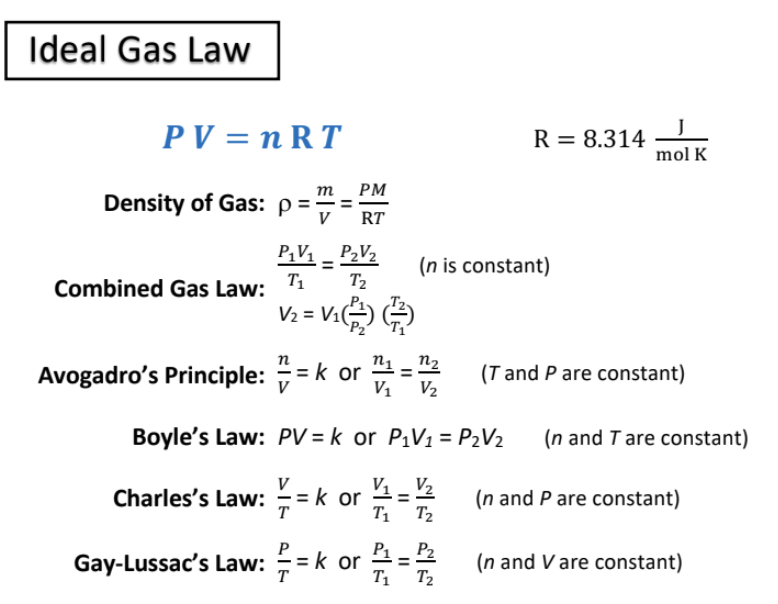
ideal gas law
4
New cards
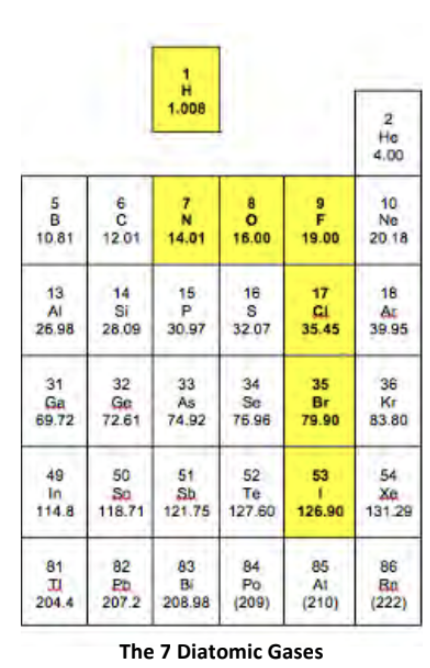
diatomic gases
H2 N2 O2 F2 Cl2 Br2 I2
have no fear of an Ice cold beer
5
New cards
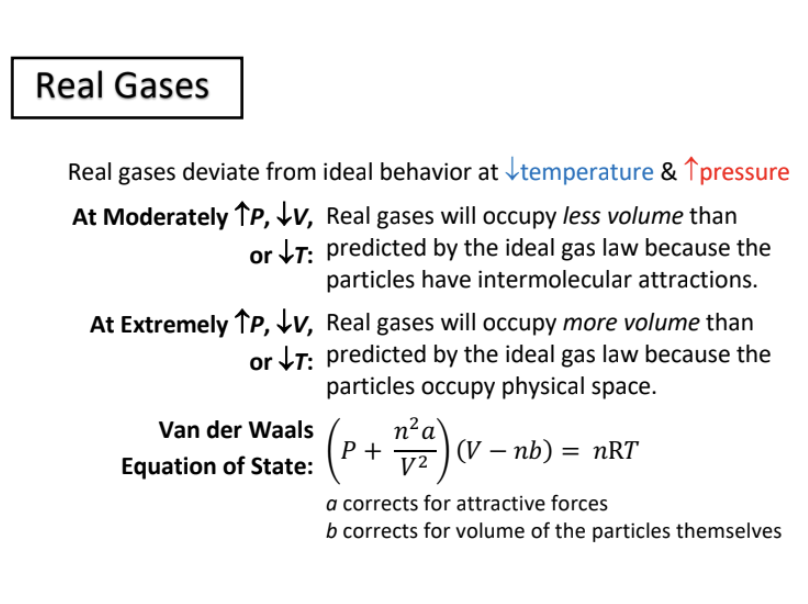
real gases
6
New cards
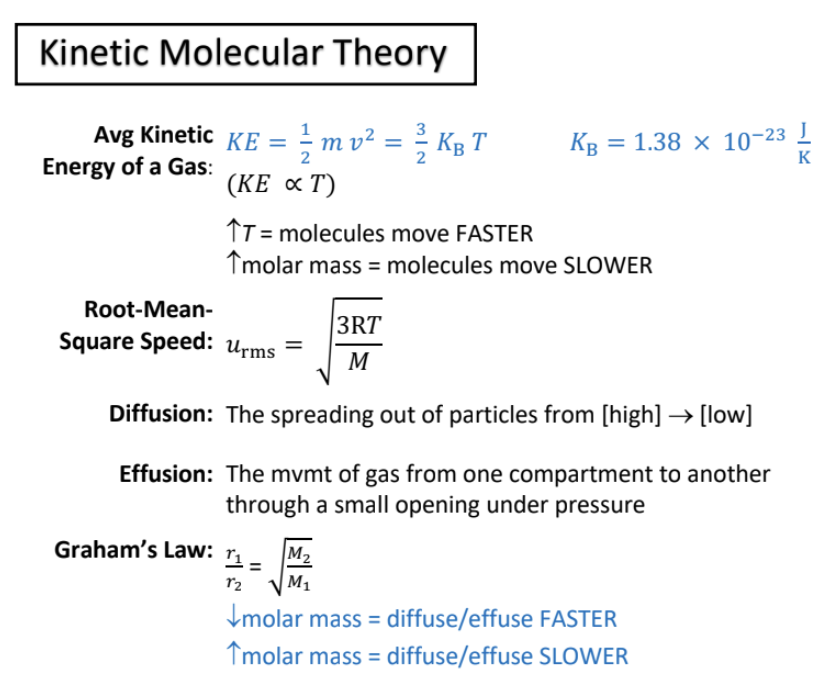
kinetic molecular theory
7
New cards
other gas laws