Chemistry lecture study for test 1
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
Mass
the amount of matter in an object (how much stuff is in the object)
Matter
a substance or solution that occupies space and has mass
Weight
The force gravity exerts on an object
Law of Conservation of Matter
Matter cannot be created nor destroyed
(Matter will have the same mass even when it has changed form)
Chemical changes
atoms recombine to form new substances
Physical substance
Matter that changes states of matter but not its composition
Elements
Pure substances that cannot be broken down into simpler forms by chemical changes
Compounds
Pure substances that can be broken down
Consists of 2+ elements that’s chemically bonded
Compound properties are different from element properties
Law of constant composition
if you take a small piece of an element, it will still have the properties of the entire element
Mixtures
Composed of 2 or more types of matter that can be present in varying amounts and can be separated in varying amounts
Homogenous (solutions) mixtures
Mixtures that are so uniform you can’t tell the matters apart
Ex. Air, coffee, brass
Heterogenous mixtures
Easy to tell one matter from another and easy to separate
Ex. oil and water, sand, smog
Pure substance can’t be…
Mixtures
Atoms
Smallest part of an element that has the properties of the element and can enter chemical combination
Molecules
2 or more atoms connected by strong forces called chemical bonds
Compounds are…
molecules but not all molecules are compounds
Ex. H2 is a molecule but not a compound because it is composed of only one element
Properties
characteristics that help distinguish one substance from another
Physical properties
Physical changes
Ex. density, color, durability, melting/boiling points and electrical durability
Chemical properties
Chemical changes, can’t change state of matter
Ex. Flammability, toxicity, acidity, reactivity, and heat of combustion
New substance after reaction
Ex. nail rusting, adding sugar to iced tea
Extensive property
Depends on the amount of matter present
Ex. mass, volume, heat
Intensive property
Independent to the amount of matter present
Ex. density, temp
Measurements provide 3 kinds of info
1) A number
2) A unit
3) An indication of the uncertainty of the measurement
SI units
Length-meter (m)
Mass-Kilogram (Kg)
Time-Second (s)
Temp-Celsius (C)
Electricity- Ampere (A)
Amount of substance- Mole (mol)
Luminous intensity-Candela (Cd)
Fractional SI units
Femto (f) 10^-15
Pico (p) 10^-12
Nano (n) 10^-9
Micro (μ) 10^-6
Milli (m) 10^-3
Centi (c) 10^-2
Deci (d) 10^-1
Kilo (k) 10^3
Mega (M) 10^6
Giga (G) 10^9
Tetra (T) 10^14
Volume
How much space is occupied by an object
Volume formula
V = m/d
Density
How heavy something is relative to its size
Density formula
D = m/v
Mass formula
m = density x volume
What is the density of water
1 gram per mL
Uncertain numbers
Quantities derived from measurements other than counting
Significant numbers
Non-zero digits
Captive zeros
Trailing zeros (when right of decimal place)
Non-zero digit
Any number other than zero
Ex. 1,2,3,4,56,64…
Captive zeros
All zeros between non-zero digits
Ex. 73.04, 500007, 9.000000006
Trailing zeros
Zeros to the right of the decimal place with no digits after them
Ex. 9.00050, 0.0000000900000
Non significant numbers
Zeros that don’t add significance to the size of the number. Usually left of the decimal point
Non significant numbers example
Leading zeros or trailing zeros with no present decimal point
Ex. 0.009, 0067, 99000
Calculation rules
1) round results to the same decimal place for +/-
2) Round results to the same amount of digits as the # with the least amount of sig. figs. for x/÷
3) for results with the last digit ≤4 round down, if ≥6 round up. If its 5 round up or down but try and make it an even number
Preciseness
When the measurement is done repeatedly and yields similar results each time
Accurate numbers
Yields results that are close to the true/accepted value
Dimensional analysis
Used to convert one unit to another unit
Conversion factor
A number used to switch units by x/÷
Dalton’s atomic theory (4 points)
1) All elements are composed of atoms
2) All atoms of the same element are identical; different elements have different types of atoms
3) Atoms can’t be subdivided, created or destroyed
4) Atoms of different elements can combine in simple whole number ratios to form chemical compounds
Law of Multiple Proportion
We can form different substances using the same elements just in different amounts
Ex. A green solid has 0.558g Cl to 1g Cu but a brown solid has 1.116g Cl to 1g Cu
Electrons
First subatomic particle to be discovered
Negatively charged, attracted to positive charges
Charge to mass ratio= 1.759 × 1011 C/Kg
Occupy almost all of an atom’s volume
Discovered by J.J Thompson using cathode rays
Nucleus
Small and dense
Contains most of the atoms mass
surrounded by electrons
The center of the atom
Discovered by Ernest Rutherford
Neutrons
Uncharged
Same mass as protons, heavier than electrons
Found in the nucleus
Discovered by James Chadwick
Protons
Positively charged
Located in the nucleus
Heavier than electrons
Discovered by Ernest Rutherford
Isotopes
Same element but different mass
Ex. Carbon 12 vs carbon 13
Caused by differing levels of protons
Written by writing the mass number as a superscript to the top left of the element symbol
Atomic number (z)
The number of protons in the nucleus
this value determines the identity of the atom
Any atom with 6 protons is carbon regardless of the isotopes
Also indicates the amount of electrons in a neutral atom
Neutral atoms
An atom with an equal number of protons, electrons, and neutrons
Mass number (A)
Total number of protons and neutrons in an atom
The number of neutrons is equal to the mass number - the number of protons
Ions
The imbalance between protons and electrons
Atom charge = # of protons - # of electrons
Atoms and molecules acquire charge by losing or gaining electrons
Anion
A negatively charged ion
Cation
A positively charged ion
Atomic mass
Each proton and neutron has a mass of ~1 amu
Electrons weigh far less
The atomic mass of one atom is roughly equal to its mass number
Mass spectrometry
The occurrence and natural abundance of isotopes can be experimentally determined using a mass spectrometer
Molecular formula
A representation of a a molecule or compound and has
1) Chemical symbols
2) Subscripts after the symbol to indicate each atom quantity in the molecule
Ex. CH4
Structural formula
shows the same things as a molecular formula plus how the molecules connect
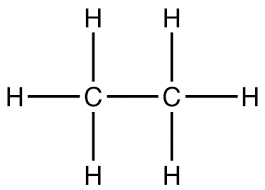
Empirical formula
The simplest way of writing IONIC COMPOUNDS (Always in whole numbers)
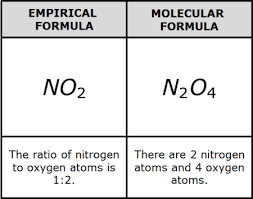
Molecular formula
Depicts the actual number of atoms in an element in the compound
MF= C6H6 Empirical formula= CH
MF= C2H4O2 Empirical formula= CH2O
Isomers
The same atom with different structures
Ex. Acetate Acid and Methyl Formate both have the same Molecular formula
Periodic law
When elements are arranged in order of increasing atomic numbers, there is periodic repetition of their chemical and physical properties
Periods or series
Horizontal rows 1-7
Groups
Vertical columns numbered 1-18
Metals
Shiny, malleable, good conductors of heat and electricity
Metalloids (semi-metals)
Conduct heat and electricity somewhat well and have some properties of metas and some of nonmetals
Nonmetals
Appear dull and are poor conductors of heat and electricity
Monoatomic ions
Only have one atom
Polyatomic ions
multiple atoms with a charge
Oxyanions
Polyatomic ions that contain an oxygen
-ate suffix means
there’s more oxygen
-ite suffix means
there’s less oxygen
Per- prefix means
Largest amount of oxygen (more than -ate)
Hypo- prefix means
Smallest amount of oxygen (less than -ite)
Ionic bonds
Transfer of electrons (losing or gaining electrons)
Non contact forces
Covalent bonds
Happens when electrons are shared and molecules form
Contact force
Ionic compounds
Metals become positive and form cations
Non metals lose electrons and form anions
Metals and nonmetals form ionic compounds
Held together by ionic bonds
Occurs a lot in transition metals or main group metals combining with a non metal
Solid with high melting and boiling points
Non-conductive in solid form
Conductive in molten form
Formulas of ionic compounds
Write the symbol and charge of the metal first and the non metal second
Swap the charges (They will be even)
Ex. Li^1 O^-2 → Li2 O
Reduce to the lowest ratio and write the new charges on bottom right of the elements
Many ionic compounds contain polyatomic ions as the cation, anion or both
Parenthesis in ionic compound formulas mean there are two or more poly atomic ions
Ex. Ca2+ and PO43- forms Ca3(PO4)2
Naming Ionic compounds with a metal ion with a variable charge
Most of the transition metals and some main group metals can form a 2+ cations with different charges
The charges of the metal ion is specified by a roman numeral in parenthesis after the name of the metal
roman numerals indicate positive charge
Older naming systems used the -ous and -ic suffixes
-ous was for the lower charge
-ic was for the higher charge
Ex. FeCl2 = Iron (II) Chloride