Physiology: Osmosis
What are the three feedback systems?
Positive (moving a variable further in the same direction of the original change)
Negative (moving a variable in the opposite direction of the original change)
Feedforward Regulation (uses cues to stimulate changes in anticipation of a change)
Mechanism of Negative Feedback + Example
Receptor detects a change in condition beyond normal specific limits; the Control center (usually the brain) evaluates the change; the Control center activates an effector (and its mechanism) to correct the condition; when the control center (brain) determines that conditions have returned to normal, the corrective action is discontinued
Example: temperature control, blood glucose concentration control
1/148
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
149 Terms
What are the three feedback systems?
Positive (moving a variable further in the same direction of the original change)
Negative (moving a variable in the opposite direction of the original change)
Feedforward Regulation (uses cues to stimulate changes in anticipation of a change)
Mechanism of Negative Feedback + Example
Receptor detects a change in condition beyond normal specific limits; the Control center (usually the brain) evaluates the change; the Control center activates an effector (and its mechanism) to correct the condition; when the control center (brain) determines that conditions have returned to normal, the corrective action is discontinued
Example: temperature control, blood glucose concentration control
Mechanism of Positive Feedback + Example
The control center evaluates the change and decides to activate an effector that will accelerate the change
Example: childbirth, oxytocin is the effector
Feedforward Regulation + Example
smell of food stimulates salivary glands to secrete enzymes; cues of fear increase adrenaline before a threat is even imminent
Compare the composition of intracellular fluid and extracellular fluid.
Extracellular Fluid (⅓) of all fluid and intracellular fluid is (⅔) of all body fluid.
Extracellular Fluid is divided into: interstitial fluid (75-80% or ¾) and plasma (20-25% or ¼ of ECF)
ECF: High Na+, Low K+ (ECF is SALTY with Na+ because it isn't INSIDE the cell!)
ICF: Low Na+, High K+
Sodium Lab Values
ECF: 140 mmol/L
ICF: 14 mmol/L
Potassium Lab Values
ECF: 4 mmol/dL
ICF: 120 mmol/L
Ca2+ Ionized Lab Values
ECF: 10mg/dL or 2.5 mmol/L
ICF: 1x10^-4 mmol/L
Cl Lab Values
ECF: 100 mmol/dL
ICF: 10 mmol/dL
HCO3- Lab Values
ECF: 24 mmol/dL
ICF: 10 mmol/dL
pH Lab Values
ECF: 7.4
ICF: 7.1
Osmolarity Lab Values
ICF/ECF: 290 mOsm/L
Glucose Lab Value
70-100 mg/dL ECF
Protein Lab Value
0.2mM ECF
Factors that influence the distribution of body fluids between ICF and ECF
Osmotic Pressure (Pull of fluid towards higher solute concentration area)
Hydrostatic Pressure (Push of fluid out of vessels into interstitial area)
Selective Permeability of Membranes
What things easily pass across the cell membrane?
fat-soluble, small particles can easily pass (O2, CO2, EtOH, fatty acids)
________ messengers can alter cell permeability, thus altering the distribution of body fluids between ICF and ECF
Chemical
endocrine, autocrine, paracrine, neurotransmitters
Endocrine
the body's "slow" chemical communication system; a set of glands that secrete hormones into the bloodstream for systemic effects
Paracrine
Referring to a secreted molecule that acts on a neighboring cell.
Autocrine
term for hormones that act on same cells that secrete them
Neurotransmitter
chemical used by a neuron to transmit an impulse across a synapse to another cell
Membrane junctions also influence cell permeability. What are the three types of membrane junctions?
desmosomes
tight junctions
gap junctions
desmosomes
strengthened by keratin, these are the strong attachments between cells by cadherin proteins; keep tissue structurally sound (e.g. skin)
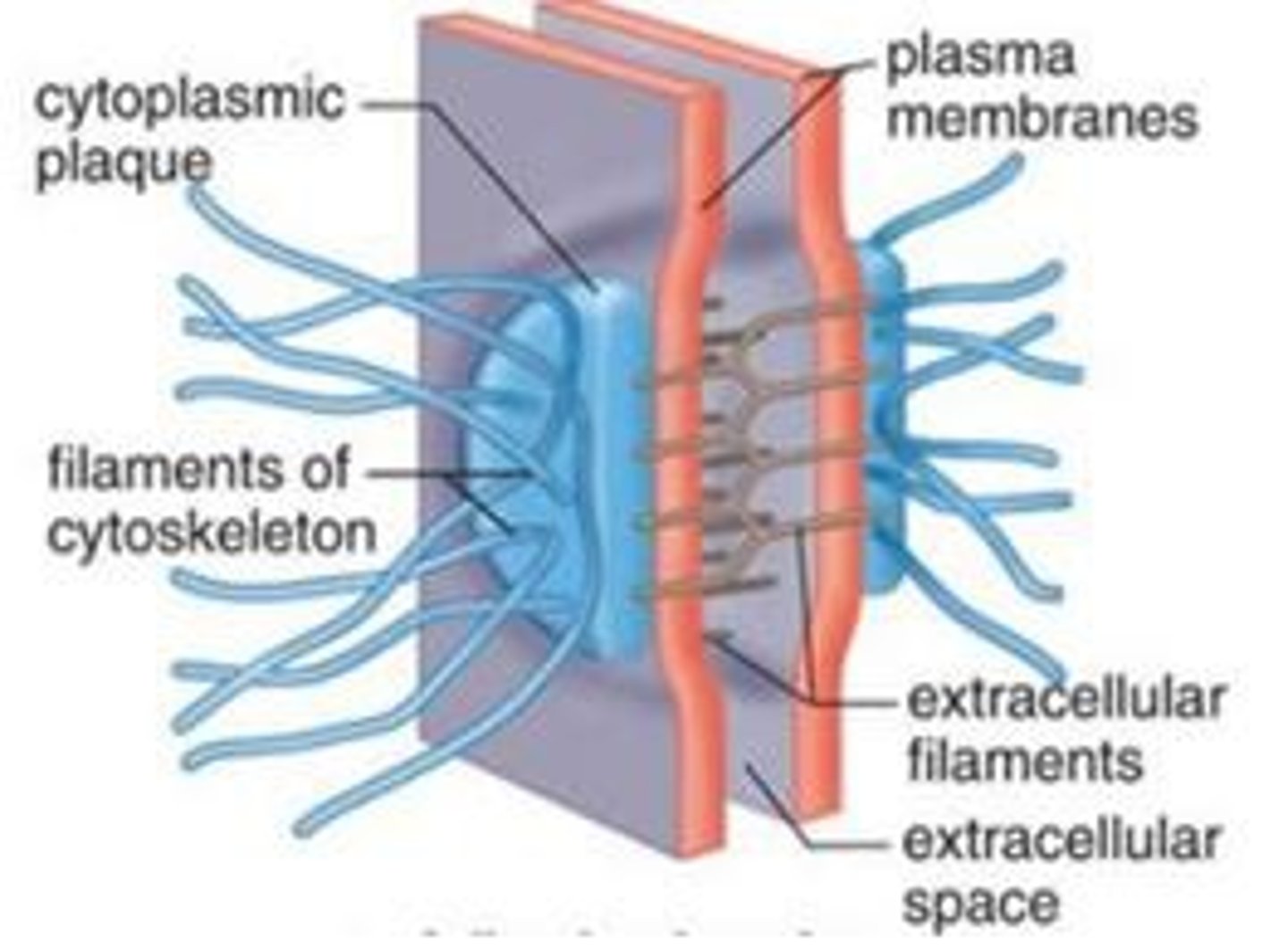
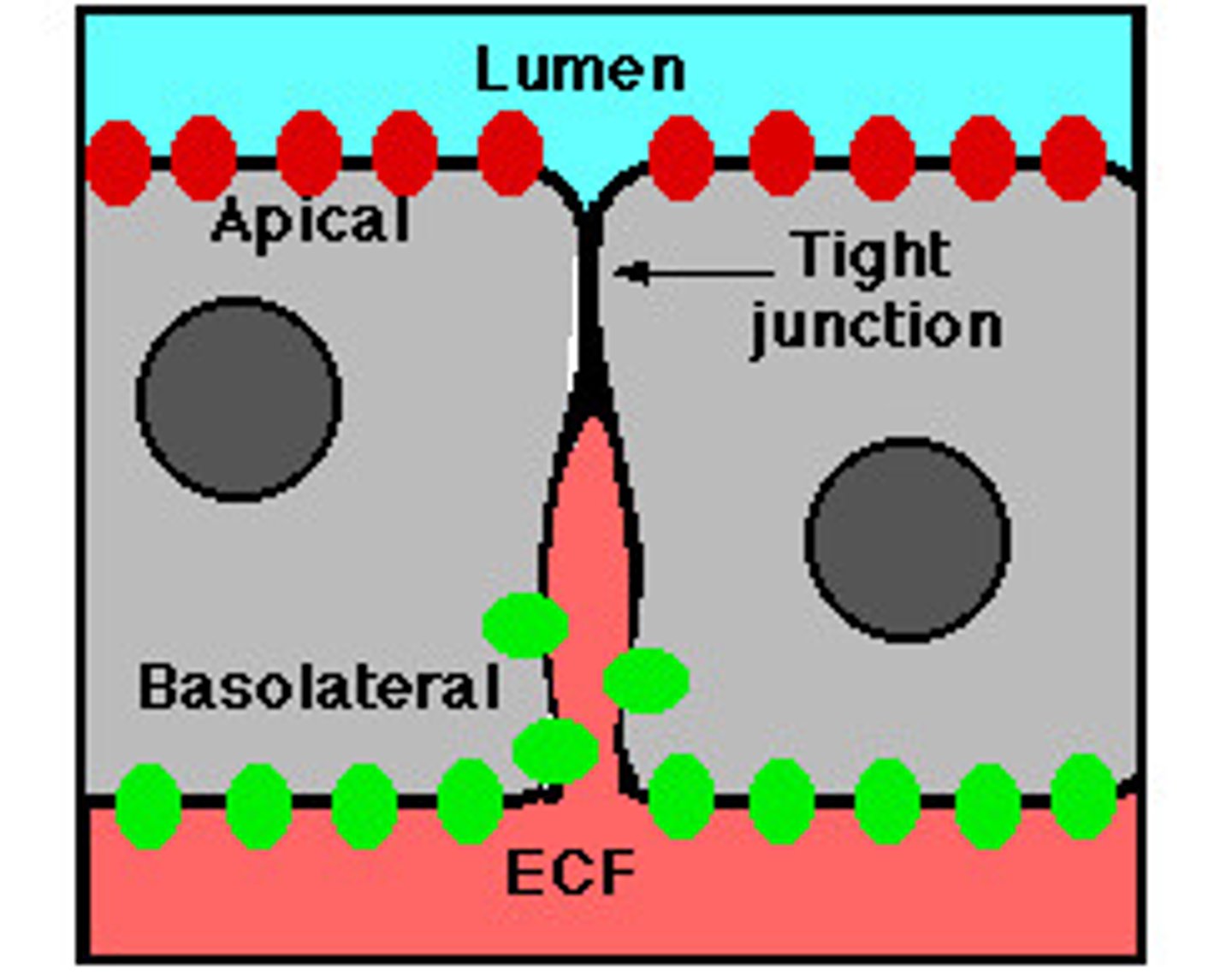
tight junctions
Membranes of neighboring cells are pressed together, preventing leakage of extracellular fluid
no extracellular space between cells (ions and water can still pass through); band around the entire cell, creating the apical (lumen-facing) surface and basal surface, forcing substances to move one-way through the cell (e.g. glucose moving through small intestine epithelial cells)
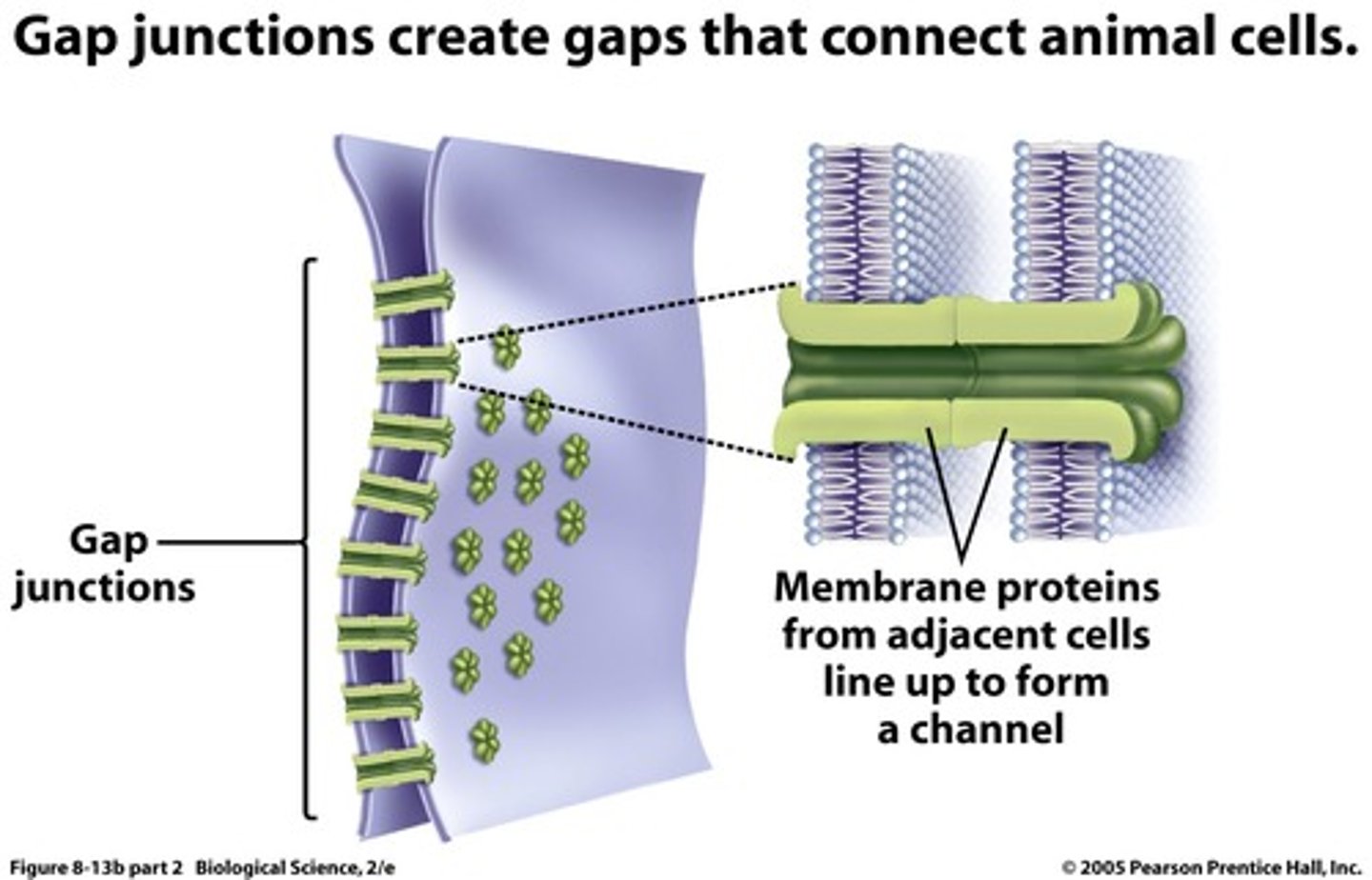
gap junctions
protein channels connect neighboring cells so that small ions can pass through the channels from cell to cell (electrical signal conduction - nerve cells, cardiac cells)
Membrane proteins also play a large role in the distribution of fluid between ICF and ECF. What are the three main types of membrane proteins?
Peripheral
Integral
Transmembrane
Peripheral Membrane Proteins
proteins associated with but not embedded within the plasma membrane; held there by weak electrostatic connections; can be disrupted with mild treatments
EXAMPLE: ankyrin protein anchors RBCs to an integral membrane transport protein
ex. Cl- HCO3 exchanger
Integral Membrane Proteins
A protein embedded in the lipid bilayer of a cell and anchored by hydrophobic interactions with cell membrane.
Examples of Transmembrane Proteins
Examples: ligand-binding receptors for hormones or neurotransmitters; transport proteins (Na+, K+, ATPase); pores, ion channels, cell-adhesion molecules; GTP-binding proteins (G proteins)
Exocytosis and Endocytosis play a large role in:
the distribution of ECF and ICF
Exocytosis
when membrane-bound vesicles in the cytoplasm fuse with the plasma membrane and release their contents outside of the cell
Exocytosis replaces portions of the _____ _____, and provides a way for membrane impermeable _____ _____ to be secreted by the cell into the ECF
plasma membrane
protein hormones
Endocytosis
when regions of the plasma membrane fold into the cell, forming small pockets that pinch off the membrane and enter the cell
reduces the plasma membrane size
In endocytosis, the membrane-bound vesicle that enters the cell has a small volume of ____ and ____ ______
ECF and Cell Membrane
Pinocytosis
cell drinking; A type of endocytosis in which endocytotic vesicles ingest extracellular fluid and its dissolved solutes and then bring that vesicle into the cell
Phagocytosis
cell eating; cell engulfs a bacteria or large particle such as debris from damaged tissues
Receptor-mediated endocytosis
certain molecules in ECF bind to specific proteins on the cell plasma membrane and enter via the protein
Rank the types of endocytosis from least specific to most specific
least specific: pinocytosis "cell drinking"
phagocytosis "cell eating"
most specific: receptor-mediated endocytosis
Passive Transport
NO ATP Required
CONCENTRATION gradient is driving force
Can be simple or facilitated (requires a carrier protein; ex: insulin, Na+, K+, Ca2+)
What factors influence the speed of simple diffusion?
Diffusion Coefficient (chances of something diffusing)
Membrane Properties (thickness, surface area, permeability)
Electrochemical Gradient
Diffusion Coefficient
The chances of something diffusing
Dependent on the molecular weight and viscosity of the solvent
A low molecular weight correlates with a ____ diffusion coefficient, meaning that molecule will encounter ___ diffusion across the cell membrane
high; easier
electrochemical gradient
The diffusion gradient of an ion, representing a type of potential energy that accounts for both the concentration difference of the ion across a membrane and its tendency to move relative to the membrane potential.
The electrochemical gradient only exists if:
the diffusing solute is an electrolyte or ion
Na+, K+, Ca2+ always have an electrochemical gradient component, and these ions can/can not diffuse into the cell freely?
they CANNOT
They always need a carrier protein
What factors influence the speed of facilitated passive transport?
Saturation
Stereospecificity
Competition
How does saturation influence the speed of facilitated passive transport?
Facilitated diffusion rate does not increase proportionally with the concentration of the solute; it flattens out eventually when all the binding sites are occupied (Transport Maximum or Tm)
Example: transport-maximum-limited glucose transport in the kidney proximal tubule
How does stereospecificity influence the speed of facilitated passive transport?
If the carrier protein channel only allows certain molecules through, then it must wait to make contact with that specific molecule.
EX: renal proximal tubule carrier protein will only bind natural D-glucose, not L-glucose
How does competition influence the speed of facilitated passive transport?
While binding sites are very specific, they may recognize and bind chemically related solutes, thus binding things that are NOT the target solute
Ex: D-galactose competes with D-glucose for the renal proximal tubule carrier protein binding sites (L-glucose isn't even in this competition because it is not stereospecific to the carrier protein in question)
Active Transport
Energy-requiring process that moves material across a cell membrane against a concentration and/or electrochemical gradient
Primary Active Transport
Active transport in which ATP is hydrolyzed, yielding the energy required to transport an ion or molecule against its concentration gradient.
ex. Na+-K+ ATPase Pump, Ca2+ ATPase Pump (PMCA pump), H+-K+ ATPase pump (proton pump)
Na-K ATPase Pump
moves 3 sodium molecules out of the cell and 2 potassium into the cell; present in all cell membranes; uses ATP as direct energy source (primary active transport); maintains the ECF and ICF composition
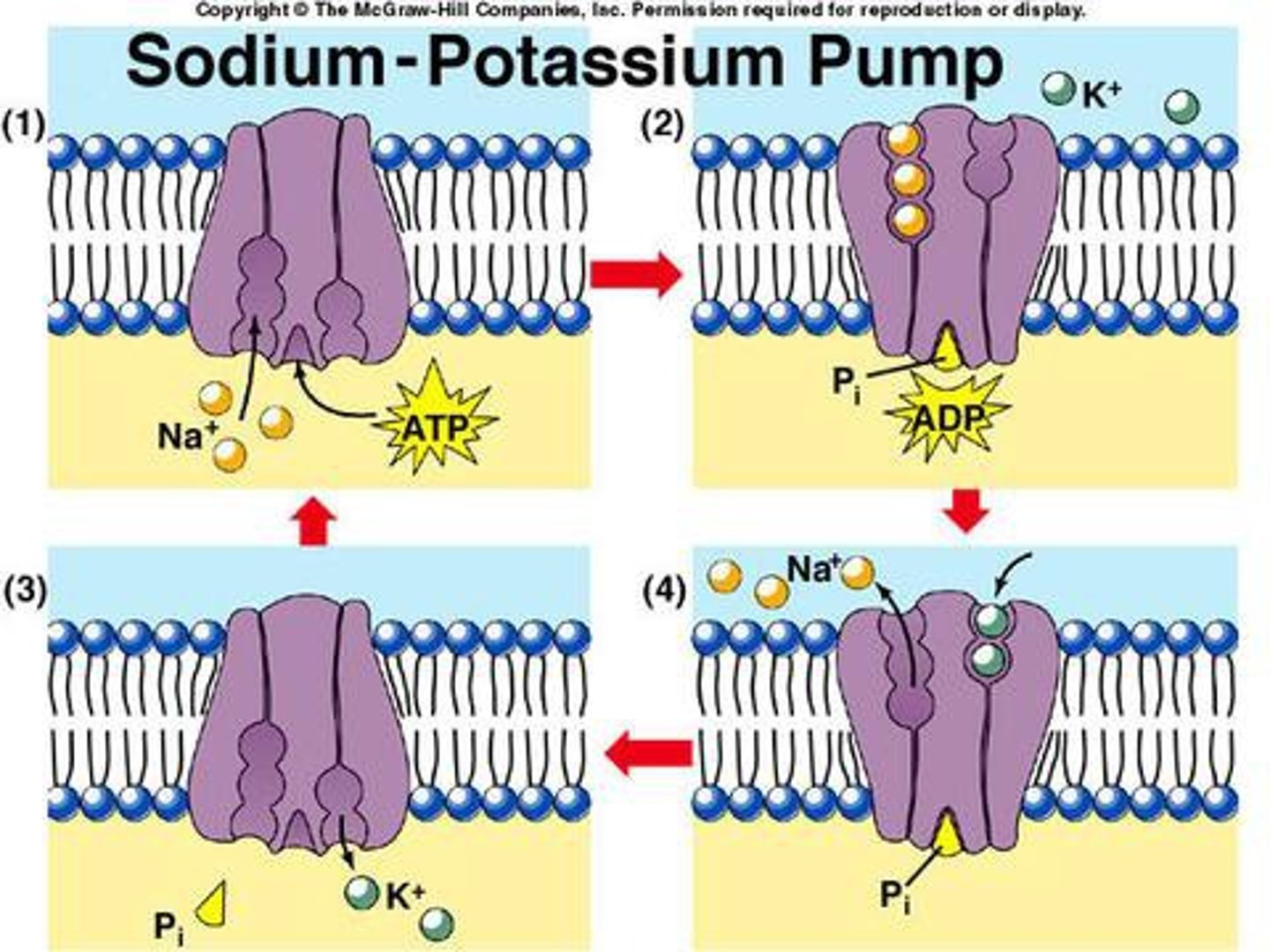
What inhibits the Na+-K+ ATPase pump?
cardiac glycosides; they cause Na to build up inside the cell
What are the two types of Ca2+ ATPase pumps?
PMCA - plasma membrane calcium ATPase pump - responsible for maintaining the very low ICF concentration of Ca by pushing Ca out of the cell against its electrochemical gradient
SERCA - pumps that sequester Ca in the SR/ER; pumps one Ca in for each ATP hydrolyzed
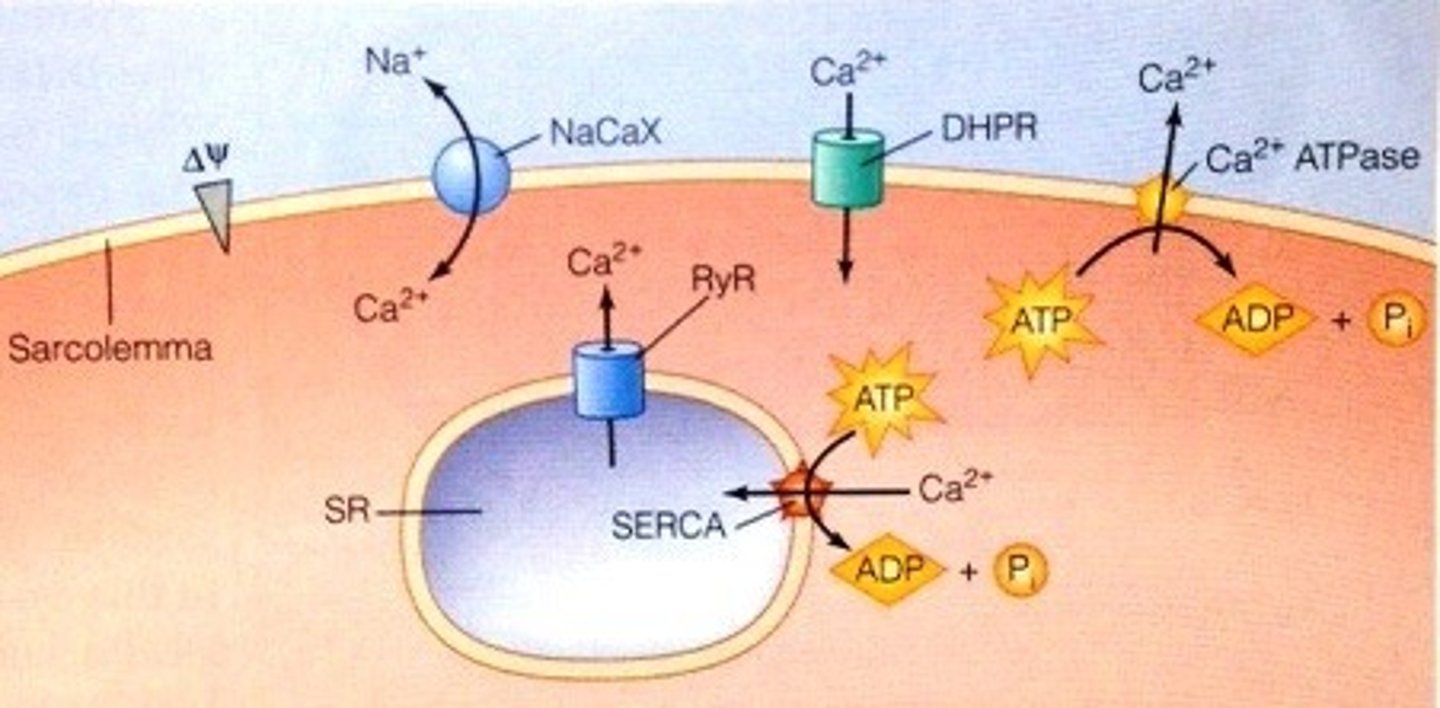
Where is the H+/K+ ATPase pump located (proton pump)?
in gastric parietal cells and renal collecting ducts (helps establish acidity)
Pumps H+ from the ICF of the gastric cells and into the lumen of the stomach, acidifying gastric contents
What does omeprazole inhibit?
proton pump inhibitor medication (PPI); it inhibits the H+-K+ ATPase pump and helps cells keep the H+ inside instead of secreting it into lumens
Secondary Active Transport
Form of active transport which does not use ATP as an energy source; rather, transport is coupled to ion diffusion (usually Na+) down a concentration gradient established by primary active transport.
Cotransport (symport)
secondary transport in same direction as Na+; the solute and the Na+ move in the same direction
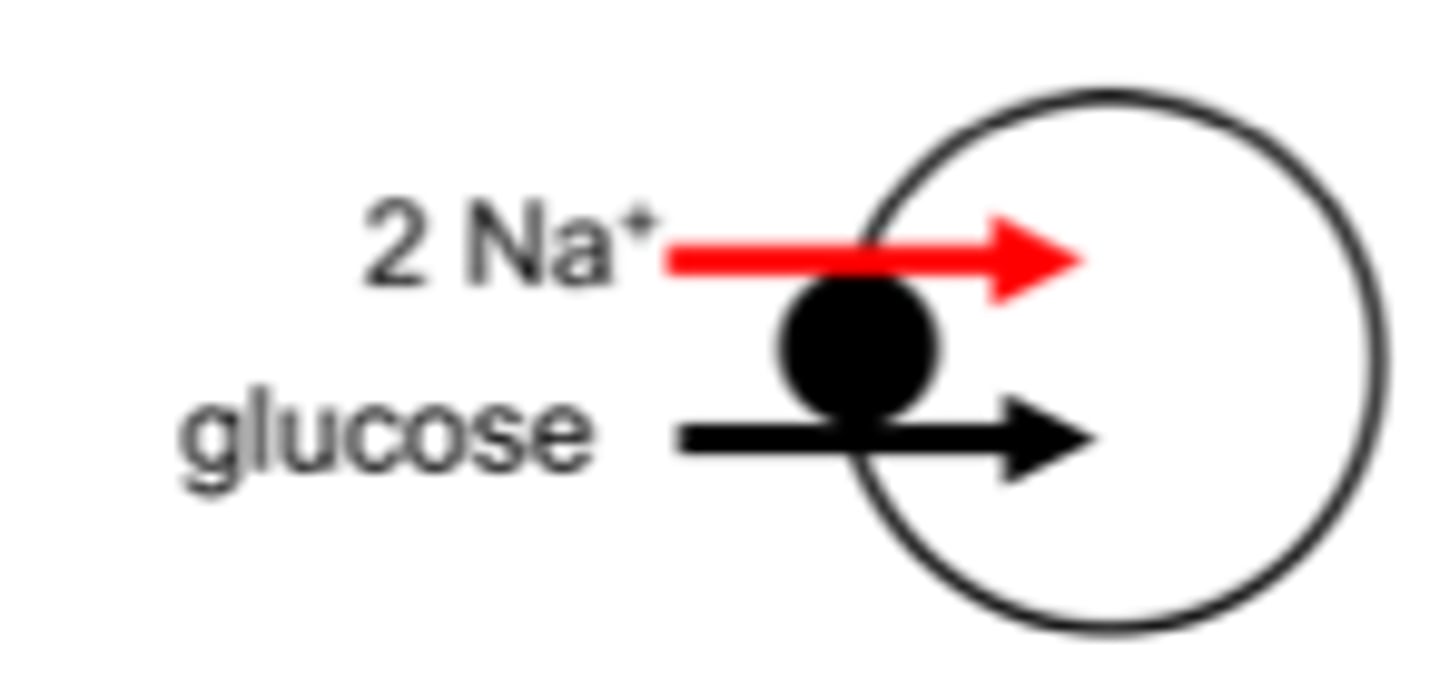
Examples of a contransport symport?
Ex. Na+-glucose cotransport pump ("SGLT") and the Na+-amino acid cotransporter in the luminal membranes of the small intestine and renal proximal tubule
The Na+-K+-2Cl cotransporter is in the thick ascending limb of the renal luminal membrane
Antiporter/Counter-transport/Exchanger
solute moving in the opposite direction of Na+ (solute moves out of the cell, while Na moves in and down its electrochemical gradient)
Example of an antiport/counter-transport pump
Ex: the Ca2+/Na+ exchanger (three Na+ comes in, while one Ca2+ moves out)
Helps maintain the low ICF concentration of calcium, along with help of the Ca2+ ATPase pump
Especially important in muscle cells, which sequester calcium in the SR
Energy for cotransport/counter-transport is provided by:
the Na+ gradient, which is established by the Na/K ATPase pump, which maintains the large ECF concentration of Na
Can a secondary active transport pump work without Na+?
No, both solutes must be present to induce the conformational change that allows the secondary active transport pump to work
Osmosis
the flow of water between 2 solutions separated by a semipermeable membrane caused by a difference in solute concentration
Are ICF and ECF separated by a semipermeable membrane?
Yes, ICF and ECF (plasma, interstitial fluid) are separated by a semipermeable membrane, the two types of ECF are also separated by a membrane (the capillary wall)
The driving force of osmosis is:
the present of a solute
Osmosis results in a ____ diffusion of water across a membrane
net
Osmosis depends on two factors:
osmolarity
osmotic pressure
Osmolarity
concentration of osmotically active particles/solutes in a solution
(mOsm/L = g*C)
g = number of particles/mol
C = concentration
depends ONLY on the number of particles/mol and the concentration of the solute.
Osmotic Pressure
pressure that must be applied to a hyper-osmotic solution to prevent the INWARD flow of water through a semi-permeable membrane
π = gCRT
includes temperature, pressure, number of particles, and concentration
Two solutions can be isosmotic, but can have different osmotic pressures due to:
variances in temperature and pressure
Isosmotic
solutions with the same concentrations of solute particles (same osmolarity)
Difference between osmolarity and osmotic pressure
osmolarity depends on particles and concentration ONLY, osmotic pressure is influenced by temperature and pressure too
Osmolarity and _____ are the same for the purposes of this course
osmolality
If the solute stays as one molecule, the osmolarity =
the concentration of the solute in the solvent
osmolarity = solute concentration in solvent
If the solute dissolves into two osmotically active particles, the osmolarity =
is greater than the concentration of solute in the solvent
Reflection Coefficient
dimensionless number ranging from 0 to 1
describes the ease of which a SOLUTE crosses a membrane
= 0 = solute crosses membrane freely, so water won't have to move (osmotic pressure unaffected)
=1 = solute does not cross membrane at all, so water must do all of the moving (osmotic pressure is at absolute maximum value)
=between 1 and 0 = water and solute may have to both move a little bit
Hypoosmotic
Solution with a lesser concentration of solute; has a lesser osmotic pressure. Has less solutes than the other solution.
Hyperosmostic
Higher concentration of solutes as compared to another (higher osmolarity); higher osmotic pressure
Example of Reflection Coefficient = 0
Ex: urea (crosses membrane very easily, so water does NOT move)
Example of Reflection Coefficient = 1
EX: albumin will NEVER cross the membrane (and should never), so a solution influenced by albumin concentration gradient will have to do osmosis to achieve equilibrium because albumin cannot cross the membrane
Osmotic Pressure is the ____ that a solution has on water. The greater the _____, the greater the osmotic pressure because the solution will have a stronger ___ for water.
Pull
osmolarity
pull
solutions that are full of osmotically active solutes will want water to come in to reduce the solute concentration; these solutions will have a strong "pull" for water and thus a higher osmotic pressure
The pressure applied to a ________ solution to stop the flow of water into that solution is the ______ ________
hyperosmotic; osmotic pressure
How does osmotic pressure work?
It pulls water into the hyperosmotic solution by reducing the hydrostatic pressure of the hypoosmotic solution via interactions with the cell membrane
Hydrostatic Pressure
Pressure exerted by a volume of fluid against a wall, membrane, or some other structure that encloses the fluid. It is the "push" on water or the pressure on water exerted by gravity. This pressure pushes water out of vessels.
Osmostic Pressure is specific to fluids _______ by a semipermeable membrane. Hydrostatic pressure is/is not?
separated
hydrostatic pressure is NOT specific to fluids separated by a semi-permeable membrane; this pressure exists with all fluids
Example of high hydrostatic pressure
High hydrostatic pressure of the glomerulus vessels in the bowman's capsule of the kidney nephron favors the filtration of water out of the vessels into the Bowman's capsule
Tonicity
the measure of the osmotic pressure gradient between 2 solutions separated by a semipermeable membrane
isotonic = same effective osmotic pressure gradient (osmotic pressure, determined by concentration of solutes + temp + pressure)
isoosmotic = same effective osmolarity (concentration of solutes)
Isotonic
when the solute concentration of two solutions is the same; same effective osmotic pressures; there is no osmosis
Hypertonic
has a higher effective osmotic pressure (higher concentration of non-permeable solutes) than the other solutions
A cell will ____ in a hypertonic solution because water will:
shrink
move out to try and dilute surrounding solution
Hypotonic
has a lower effective osmotic pressure (lower concentration of non-permeable solutes) than others
Cell will ___ in hypotonic solutions because water will:
swell; move into the cell to try and dilute the relatively high number of solutes in the cell
What are the symptoms of fluid deficit/dehydration (9)? Recall, this can be caused by hyperosmotic or isoosmotic fluid contraction.
Thirst
Weight Loss
Dryness of mouth, throat, face
Absence of sweat
Increased body temp
Low urine output
Postural Hypotension
Dizziness and Confusion
Increased Hematocrit
What are the two causes of ECF fluid deficit/dehydration?
Loss of water without the loss of solutes
Loss of water AND solutes
Loss of water without loss of solutes from the ECF will make your ECF ____ and _____
hyper-osmotic and hypertonic
What happens when you lose water but not solutes?
Water loss without solute loss from the ECF results in excess concentration of body solutes within the interstitial and vascular compartments both.
In efforts to preserve equilibrium, water will shift out of cells and into the ECF, causing cells to shrink.
The volume of the ECF will then increase, and if this pattern persists, the water will be excreted by the kidneys by osmotic diuresis leading to severe cellular dehydration.
Osmotic Diuresis
the increase in urine output caused increased solute concentration in the ECF, causing the movement of water out of cells and into the ECF to dilute the solutes
Causes of dehydration due to water loss (and no solute loss):
1. Decreased water intake
2. Water loss without proportionate solute loss (hyperventilation, diabetes insipidus)
3. Increased solute intake without proportionate water intake (tube feeds)
4. Excess accumulation of solutes (high blood sugar due to DM)
5. Loss of excessive sweat (hypoosmotic solution compared to ECF-mainly water with very few solutes)
6. Hyperosmotic volume contraction (vessels clamped down)
Hyperosmotic volume contraction
refers to the loss of free water greater than electrolytes which can be seen in diabetes insipidus and in states of excessive sweating without fluid replacement
Dehydration due to water loss (without proportionate solute loss)