Hess’ law and enthalpy cycles
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
What does Hess’ law state?
If a reaction can take place by 2 routes, and the starting and finishing conditions are the same the total enthalpy change is the same for each route
2
New cards
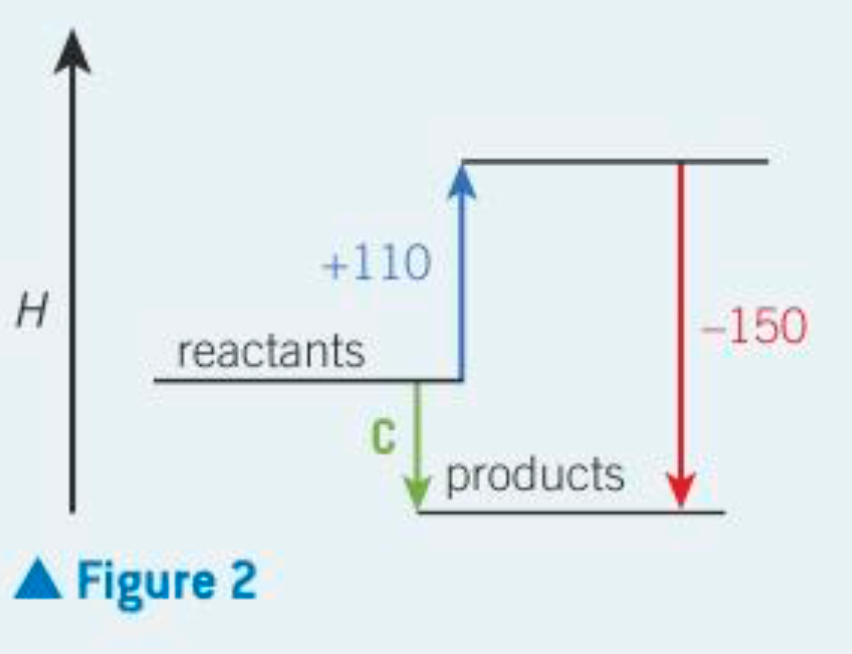
How would you work out c?
A + B = C
110 +- 150 = -40
3
New cards
How do you construct an enthalpy cycle from ΔfH?
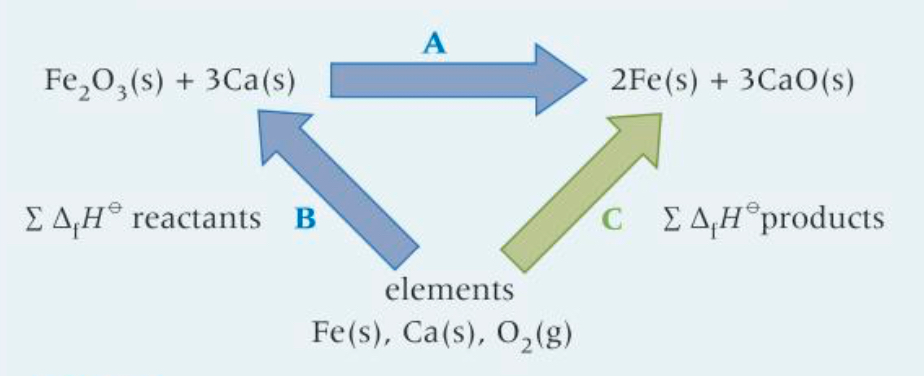
4
New cards
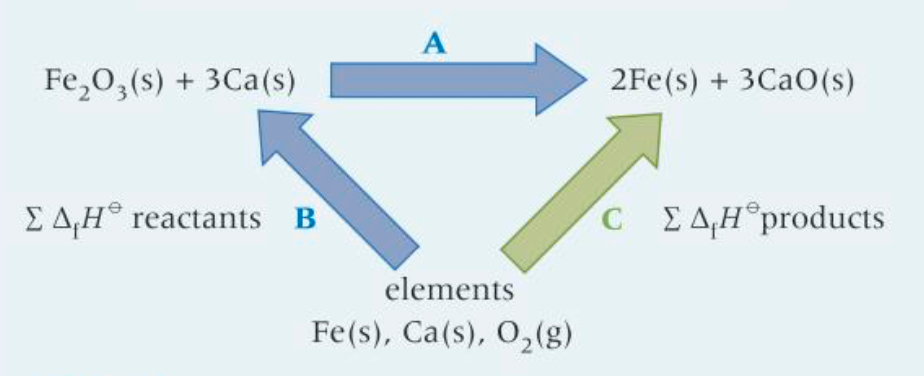
How would you calculate A?
-B + C
5
New cards
How do you construct an enthalpy cycle for ΔcH?
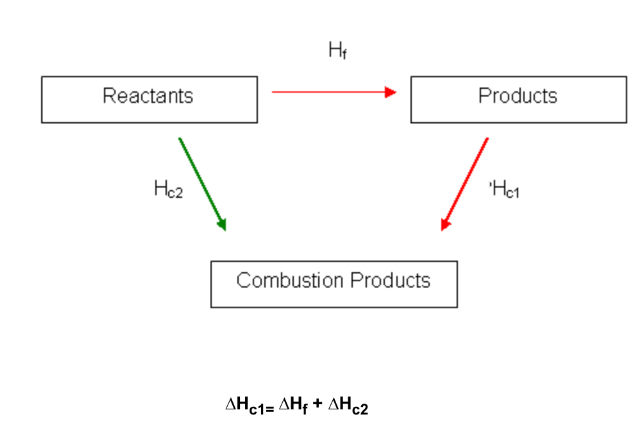
6
New cards
When calculating a you should start from which end of the arrow?
Opposite end (not the arrow head)