T3:Particle model of matter
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What is the formula to calculate the density of a material(include units)?
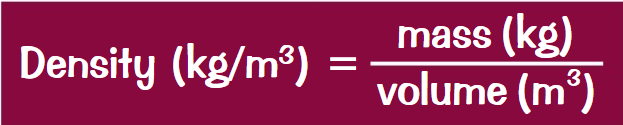
What are the properties of a solid?
Strong forces of attraction holding the particles close together in a fixed,regular arrangement.
Particles vibrate about fixed positions
Highest density
What are the properties of a liquid?
Weak forces of attraction holding the particles close together in an irregular arrangement
Particles move in random directions at low speeds
Medium denisty
What are the properties of a gas?
Almost no forces of attraction between particles
Particles move in random directions at high speeds
Low density
Draw a diagram to represent a solid:

Draw a diagram to represent a liquid:
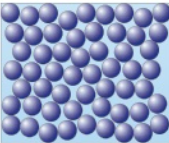
Draw a diagram to represent a gas:
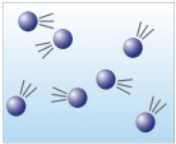
Why are changes of state physical changes and not chemical changes?
The material recovers its original properties if the change is reversed.
Why is mass conserved in a state change?
The number of particles do not change.
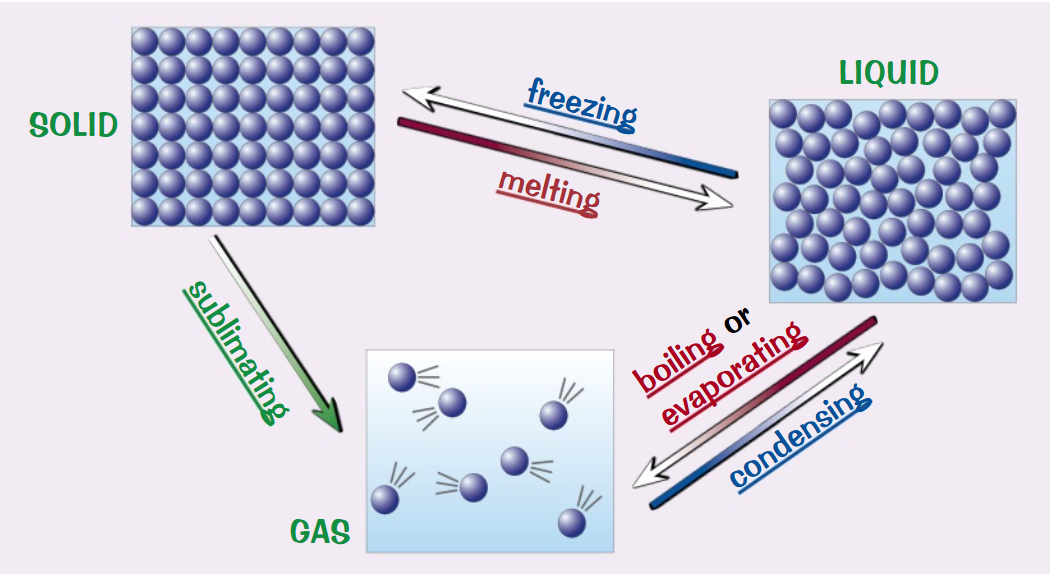
Draw a diagram to represent the changes in state(use words and arrows only):
What is energy stored by in a system?
The particles that make up the system.
What is the internal energy of a system?
The internal energy of a system is the total energy that its particles have in their kinetic and potential energy stores.
What does heating the system do?
Heating the system transfers energy to it’s particles as they gain energy in their kinetic enrgy stores and move faster , increasing the internal energy.
What two things can raising the temperature in a system cause?
raises the temperature of the system
causes a change in state
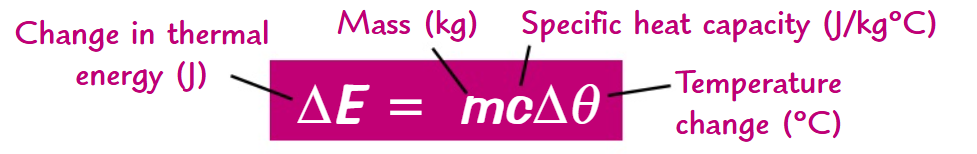
What does the thermal energy of a substance depend on?
The mass of the substance heated
They type of material
The energy input into the system
What is the specific heat capacity of a substance?
The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1kg of the substance by 1°C.
what is the formula to calculate the specific heath capacity of a substance(include units)?
What is latent heat?
the enrgy needed for a substance to change state.
When a change of state occurs, the energy supplied changes the energy stored but not the…
Temperature.
What is the specific latent heat of a substance?
The specific latent heat of a substance is the amount of energy required to change the state of 1Kg of the substance with no change in temperature.
What is the formula to calculate the specific latent heat of a substance(include units)?
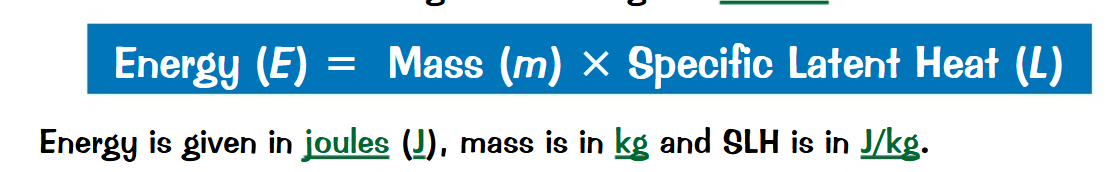
What specific latent heat is the change of state from solid to liquid?
Specific latent heat of fusion.
What specific latent heat is the change of state from liquid to vapour?
Specific latent heat of vaporisation.
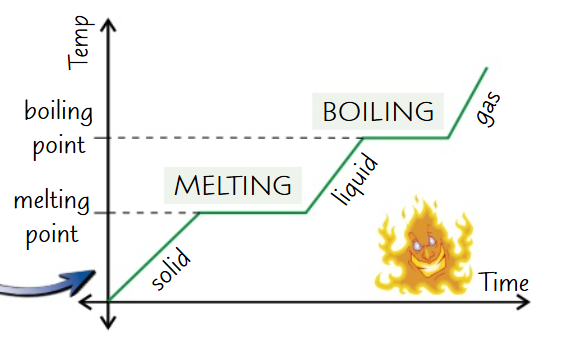
Draw and label a heating graph:
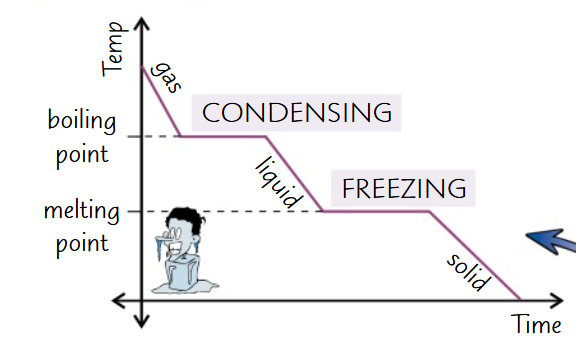
Draw and label a cooling graph:
Are molecules opf gas in constant random motion?
Yes.
What is the temperature of a gas related to?
The average kinetic energy of the molecules.
What happens as you increase the temperature of a gas?
The average speed of its particles increases.
What happens when youchange the temperature of a gas ,held at a constant volume?
Changes the pressure exerted by the gas.
If the gas is compressed too quickly the temperature of the gas increases. Explain how the temperature increase would affect the pressure exerted by the gas.
( temperature increase would increase the pressure in the tube
because a higher temperature would mean higher average kinetic energy of particles.
What happens when a gas is compressed or expanded by pressure changes?
The pressure produces a net force at right angles to the wall of the gas container.
Why does increasing the volume in which a gas is contained at a constant temperature lead to a change in pressure?
Increasing the volume of a gas means
the particles get more spread out
and hit the the walls of the container less often
so the gas pressure decreases
What is the formula to calculate the pressure for a gas of mixed mass and at a cnonstant temperature9include units)?
Pressure=Pascals(Pa)
Volume=cm³
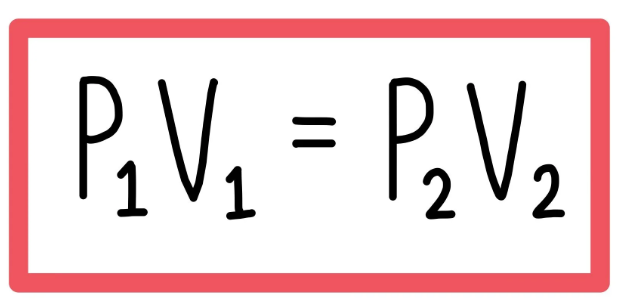
What is the relationship between pressure and volume and what does it mean?
Inversely proportional
When volume goes up,pressure goes down.
What is work?
Work is the transfer of energy by a force
What does doign work on a gas do?
It increases the internal energy of the gas and can cause an increase in the temperature of the gas.