1,2,3 Ceramics
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Define Ceramics
What type of bonding
Occur as
Chemical mixture of metallic & non-metallic elements, formed at high temperatures
Bonding is a combo of ionic & covalent
Occur as minerals, O, Si and Al, 85% of the earth’s crust
Ceramics Physical, chemical, mechanical and biological properties
Physical: Intermediate density, high melting point, low coefficient of thermal expansion (1-15ppm/C), High modulus (E) / stiffness
Chemical: Low chemical reactivity, low absorption, low solubility
Mechanical: 10x stronger in compression than tension but brittle, low fracture toughness, poor fatigue resistance.
Biological: Relatively inert, excellent biocompatibility, sometimes bioactive
General Advantages & Disadvantages of Ceramics for Biomedical Use
Advantages: chemically inert, bioactive in body, high weight resistance, high modulus (stiffness) and compressive strength, esthetic for dental applications (can be translucent, ZrO2, opaque)
Disadvantages: Brittle, Low tensile strength, Poor fatigue resistance, difficult to fabricate and expensive
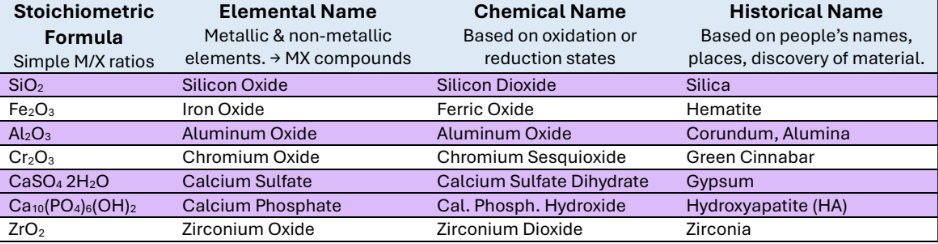
Historical names: Silica, Hematite, Corundum/ Alumina, Green Cinnabar, Gypsum, Hydroxyapatite (HA), Zirconia
Stoichiometric formula
Elemental Name
Chemical Name
Define Glass
Glass is an inorganic product of melting something (fusion) and then cooling it to a rigid state quickly without allowing it to solidify
Amorphous: lacking lattice structure/ crystallinity and possessing only short-range atomic order AKA glassy or vitreous

Define glass-ceramic
A controlled, partial crystallization of glass that yields a semi-crystalline solid → better translucency and machinability than fully crystalline
Partly amorphous: meaning it has some crystals/lattice structure
Ex: porcelain and emax
Define Dental Porcelain
Glass ceramic → vitreous (semi-crystalline) + Potassium oxide alumina silica/ K2OAl2O3-SiO2
Derived from the thermal processing of Quartz, Feldspar and Clay
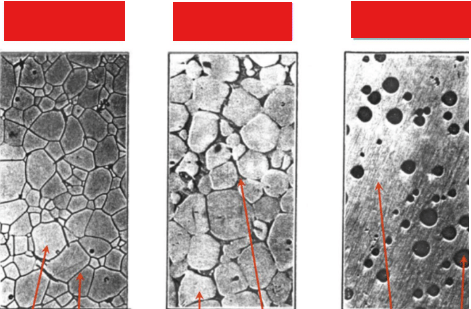
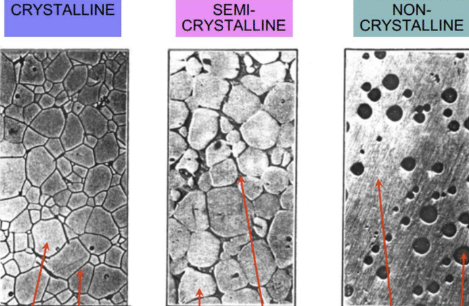
Characteristics that make ceramic crystallization difficult, and label pictures
Thermodynamic considerations: heat maximizes packing efficiency and coordination number
Kinetic considerations: affects symmetry, requires heat and time
Mechanisms of Crystallization: Nucleation and growth is slower due to large critical radius size and complicated symmetry
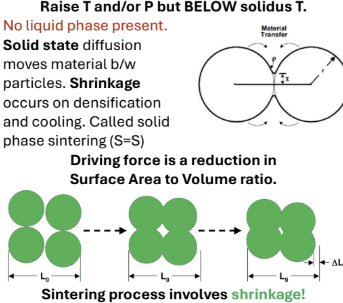
Densification
The process of forming a coherent solid from particles by melting, vitrification or sintering
Melting: cant be done to ceramics with high melting temps
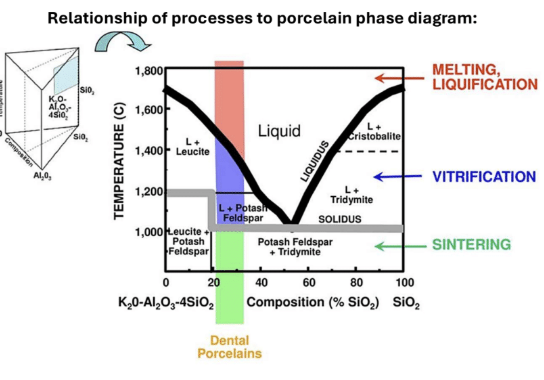
Vitrification: temperature between liquid and solid temp that causes the melted liquid portion flows around the unmelted portions and fills gap, thus strengthening the material

Sintering: temperature lowered below solidus temp, which causes shrinkage and decreases SA to Volume ratio (increases density)
Solid state diffusion
Ex: zirconia

How does porosity affect strength in a ceramic?
Ceramics can lose 70% strength at 10% porosity
Can be the result of incomplete sintering
Volumetric defects such as voids, pores, etc are key to mechanical behavior
Relationship between thermal expansion, melting point, and modulus
High melting point, low thermal conductivity, low CTE (1-15ppm)
High Modulus (E) → very stiff
Melting point and Modulus are usually proportionate
Most ceramics are anisotropic, meaning properties depend on crystallinity and temperature (measurement direction)
Chemical reactivity of most ceramics
Very limited reactivity, relatively inert and resistant to all acids except HF
HF is a weak acid but F- is super reactive
Not subject to corrosion
Fluoride treatments can etch dental ceramics like porcelain
Dental porcelain basic composition and uses
ALL are glass-ceramics and composed of Silica (SiO2), Alumina (Al2O3) and Potassium Oxide (K2O)
Used for veneers, posterior/anterior crowns, bridges, FPDs
Explain the CTE mismatch in PFM (porcelain-fused-metal)
CTE for Metal is slightly higher (so it contracts) than porcelain (1-15) so upon cooling after heating, the metal places porcelain on compression
Metal wants to shrink but cant because porcelain is bonded on top
Porcelain is stronger 10x in compression than tension so pre-loading it makes it harder for cracks to open
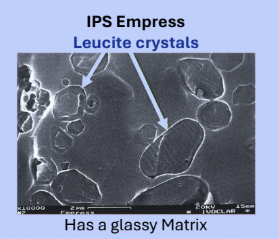
Feldspathic porcelain
Traditional porcelain, 15-20% Volume of leucine crystals, glassy matrix
Only used for veneering, not monolithic
Leucite-Reinforced Porcelain
+ ex.
35-45% Leucite crystals by volume, makes it 50% tougher (resistance to defect propagation) than Feldspathic
Ex. IPS Empress, good for veneering and improved strength
Lithium disilicate (ceramic material)
+ ex.
Glass ceramic; has 70% Lithium disilicate (Li2Si2O5) which are rod shaped crystals that block crack propagation
Balance between esthetics due to glassy matrix and durability
Used monolithically or veneered
Ex. IPS, Emax

Partially Stabilized Zirconia (PSZ)
Explain its phase transformation
Explain fabrication
Dentistry uses
Explain Monolithic Zirconia
Explain High Translucency Zirconia
Stable form of zirconia with the highest fracture resistance of all ceramics because they are durable and fatigue resistant
Phase transformation: from tetragonal to monoclinic associated with 4-5% volumetric expansion
Fabrication thru sintering, gets 25-30% shrinkage
Used for everything; crowns, bridges, implants, abutments
Very tough but ofc no as tough as metals, and do not produce significant wear on opposing natural teeth
Monolithic zirconia: the entire crown is zirconia. eliminates problem where veneering porcelain layer would fracture
Disadvantage is that it has very limited optical properties, they are also hard to adjust due to complete crystallinity after sintering
Most popular in the US
High Translucency zirconia: mix of cubed (transparent) and tetragonal zirconia; improved esthetics but decreased fracture strength
Must me 0.5mm or less, no sharp angles
CAD/CAM advantages and disadvantages
Advantages: speed, reproducibility, elimination of physical impressions, improvement of dimensional accuracy, warns if prep is under-reduced → Best for high volume bc of cost
Disadvantages: expensive, must have high volume
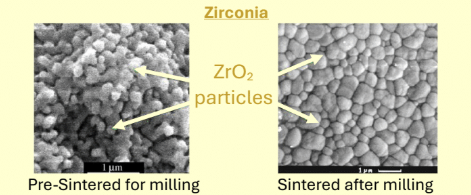
Zirconia milling caveat with CAD/CAM
When milling zirconia, there is partial sintering of the block previously and then there is fully sintering so CAD design must compensate for shrinkage
What contributes to clinical failures with ceramics?
Material limits, poor design (sharp angles, thin, etc.), adjustment with handpiece (introduction of flaws)