Acids and Alkalis
1/10
Earn XP
Description and Tags
https://www.youtube.com/watch?v=ZWZTDiwOWiI&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=5
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
What is aq
Its an aqueous solution, which means its dissolved in water. Its the symbol next to acids.
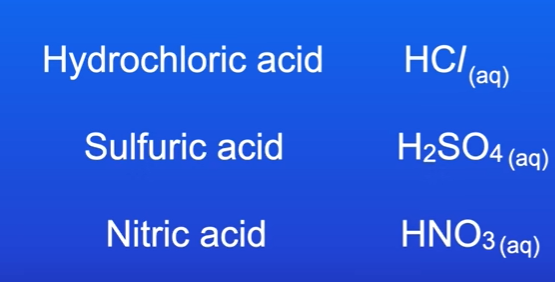
What are 3 commonly used acids.
Hydrochloric acid Hcl
Sulfuric Acid H2SO4
Nitric acid HNO3s
Whats a key fact about acids
Acids produce hydrogen ions (H+)
What are bases
Chemicals that can neutralise acids, producing a salt and water.
Bases are usually metal oxides or metal hydroxides.
What are examples of bases
Copper oxide, Iron (III) hydroxide and sodium hydroxide.
These are all bases as they can neutralise acids and produce a salt and water
What are alkalis
Bases that are soluble in water.
In aqueous solutions, alkalis produce hydroxide ions (OH-)
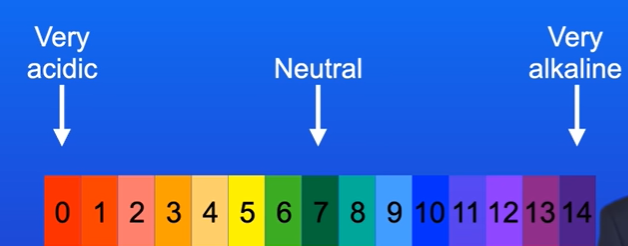
What does pH scale tell us
The acidity or alkalinity of a solution
Acids have a pH of 0-6.
Solutions with pH 7 are neutral.
Alkali solutions have pH 8-14.
How can we determine pH of solution
By using pH probe or universal indicator.
pH probe detects pH electronically.
Universal indicator changes colour depending on wether a solution is acid alkali or neutral.
What do different colours on universal indicator present
Green is neutral
Red is very acidic
Purple is very alkaline.
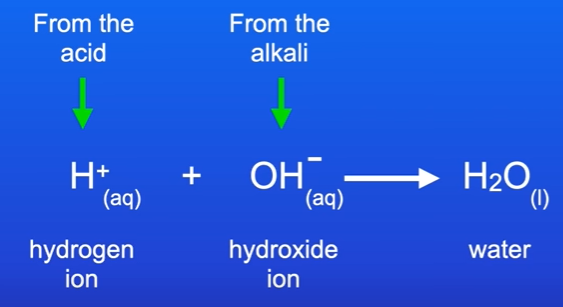
What happens when we react acid and alkaline
Hydrogen atoms come from acid, and hydroxide ions come from an alkali.
The hydroge and hydroxide ions reacft to produce water.
This is equation for neutralisation.