Covalent bond stuff and chapter 8
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
tetrahedral
AX4 e- domain geometry
tetrahedral
AX3E e- domain geometry
tetrahedral
AX2E2 e- domain geometry
trigonal planar
AX3 e- domain geometry
linear
AX2 e- domain geometry
tetrahedral
AX4 molecular geometry
trigonal pyramidal
AX3E molecular geometry
bent
AX2E2 molecular geometry
trigonal planar
AX3 molecular geometry
linear
AX2 e- domain geometry
linear
AX2 molecular geometry
109.5
AX4 bond angle
107
AX3E bond angle
104.5
AX2E2 bond angle
120
AX3 bond angle
180
AX2 bond angle
AX4
CF4, CH4, CBr4
AX3E
NH3, PCl3, NF3
AX2E2
H2O, OF3, SCl2
AX3
BF3, BH3, also C=C
AX2
CO2
sigma
single bond
pi
double bond
orbital hybridization
mixing of the atomic orbitals to form new bonding orbitals
SP3
anything with tetrahedral electron domain geometry (AX4, AX3E, AX2E2) has a hybridization of:
SP3
most single bonds have a hybridization of:
SP2
trigonal planar e- domains have a hybridization of:
SP2
boron compounds and double bonds are:
SP
linear e- domain has a hybridization of:
SP
triple bonds and CO2 have a hybridization of:
triple
one sigma, two pi
double
one sigma, one pi
single
one sigma
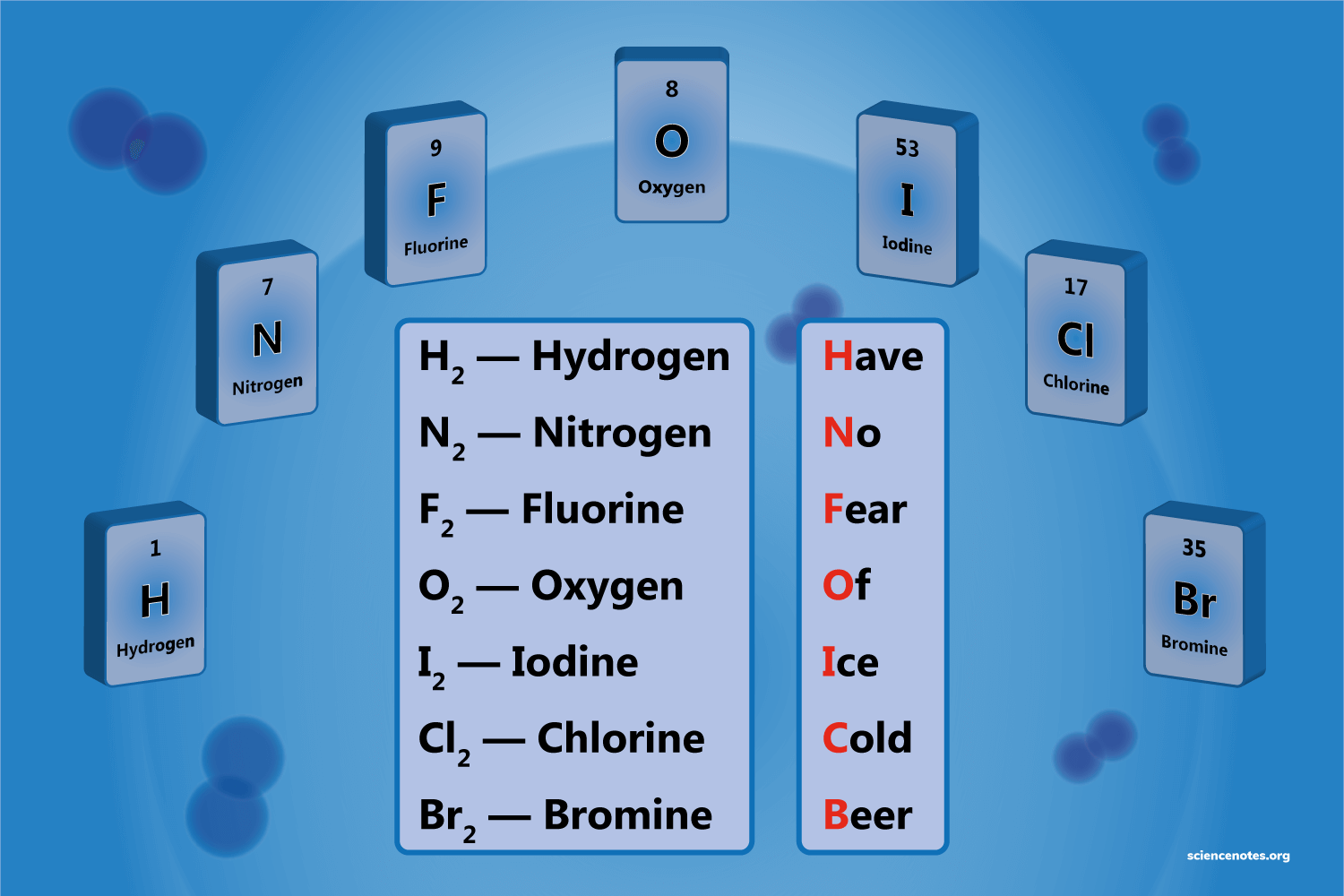
H,N,F,O,I,Cl,Br
diatomic elements
intermolecular forces
attractive forces between covalent molecules, much weaker
LD forces
all molecules have this, but this is the only one NONPOLAR molecules have
LD forces
the motion of electrons creates a temporary dipole
more electrons
LD forces are stronger the ________ you have
DD interactions
must have polar molecules, stronger than LD forces
DD interactions
create permanent dipoles
hydrogen bonding
strongest IMF
F, O, N
in a hydrogen bond, H is attached to:
non polar bond
electrons are shared equally between two atoms
non polar
molecules of the same atom, or C and H are:
polar bond
unequal sharing of electrons between 2 atoms, electrons are attracted to the more EN atom
polar
if two atoms are different, the bond is:
non polar molecule
non net dipole, molecules with only NP covalent bonds and polar bonds that cancel out
polar molecule
net dipole, lone pair on central atom
resonance
2 or more valid Lewis structures are possible, must have at least one double/triple bond in the possible lewis structure(ex. O3)