MMSC433 Exam 1 (rough)
1/139
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
140 Terms
describe how iron is absorbed in the body
ferric iron is taken in from diet
ferric iron is reduced by duodenal cytochrome B to become ferrous iron
ferrous iron is absorbed into enterocytes by DMT 1
absorbed iron is stored as ferritin, or sent into portal hepatic circulation and carried by transferrin to developing RBCs
where is iron absorbed in the body?
duodenum and upper jejunum
which of the following represents ferrous iron?
Fe2+
which of the following represents ferric iron?
Fe3+
transferrin
plasma carrier protein for ferrous iron
high iron level regulation
hepcidin is released from hepatocytes
ferroportin is inactivated, leading to decreased iron being transported into circulation
low iron level regulation
hepcidin is down regulated by hepatocytes
ferroportin becomes activated and transports iron out of the enterocytes and into circulation
hepcidin and ferroportin are:
inversely proportional
dietary iron sources
red meat, legumes, dark leafy vegetables, whole grains
which of the following is NOT a dietary source of iron
dairy products
ferrous iron
the form of iron that is able to be utilized in the body for developing red cells
stain that is used to identify iron in tissues and bone marrow
prussian blue stain
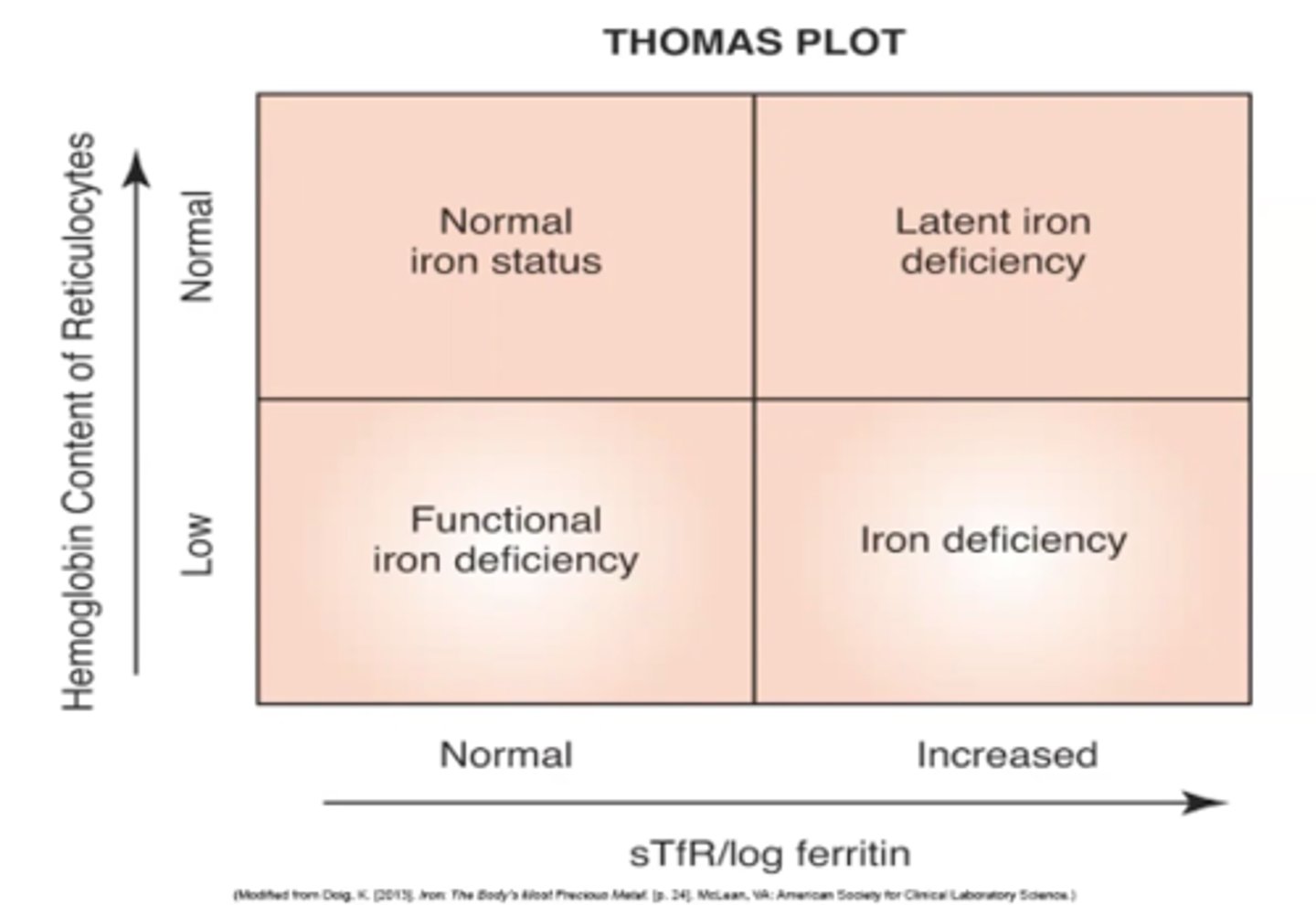
thomas plot
a chart that is used to compare soluble transferrin receptors/ log ferritin to hemoglobin concentration of reticulocytes to identify the iron status of the patient
-iron status is used to correlate to certain diseases/anemias
stage 1 iron deficiency (progressive loss of storage iron)
-asymptomatic
-RBCs develop normally
-serum ferritin low
stage 2 iron deficiency (exhaustion of iron storage pool)
-subclinical symptoms
-hemoglobin in retics is decreased, hemogram appears normal still
-iron deficiency erythropoiesis is occurring
-hepcidin decreased
-serum iron and ferritin decreased
-RDW, TIBC and sTRs increased
-prussian blue stain of BM is negative for iron
stage 3 iron deficiency (frank anemia)
-patient exhibits fatigue, weakness, pallor, glossitis, koilonychia and pica
-H/H decreased
-hypochromic/ microcytic anemia
-FEP, TIBC and sTR increased
-ferritin, hepcidin and serum iron decreased
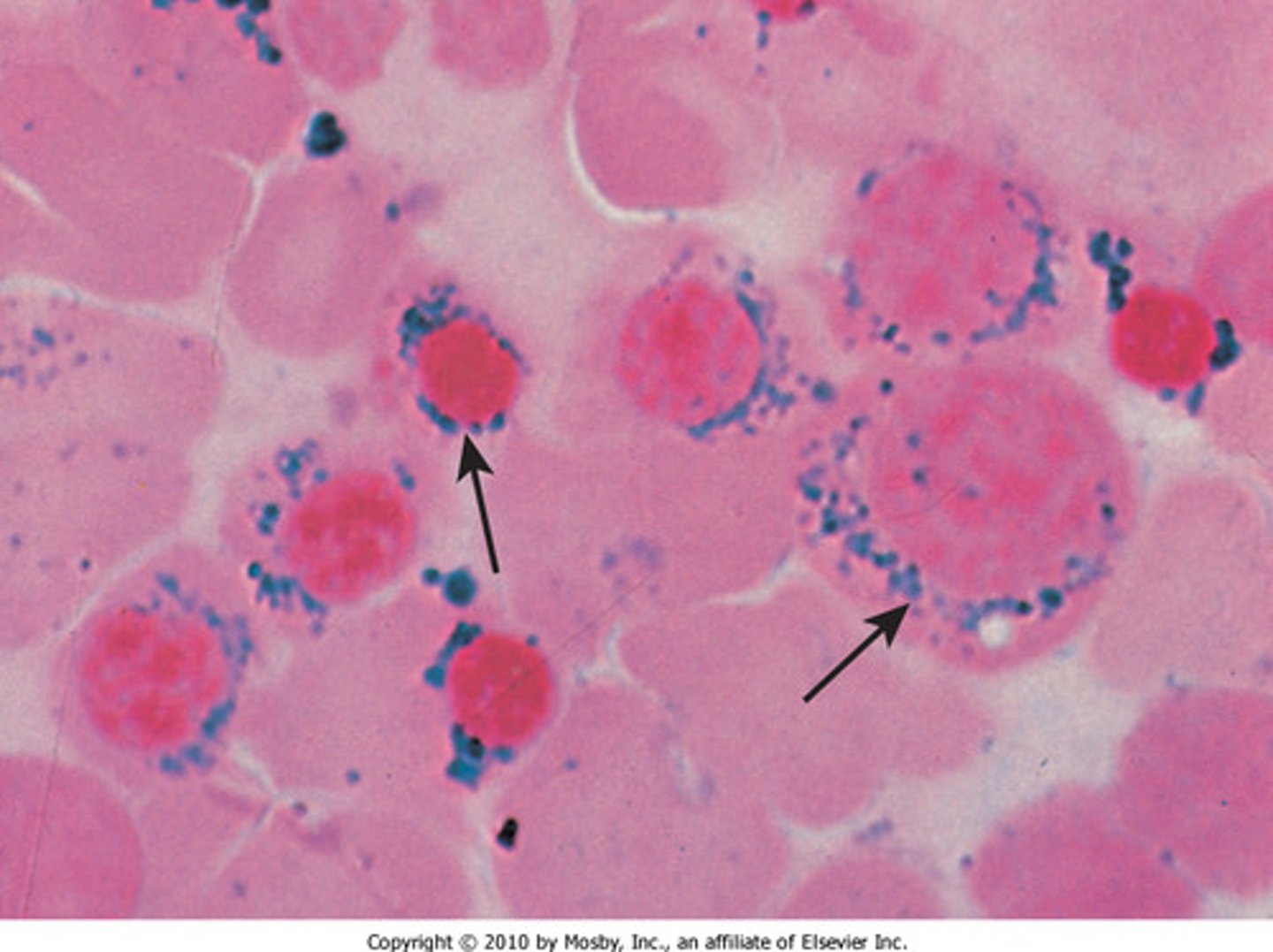
sideroblastic anemia
-iron deposits in the mitochondria of erythroblast cells in the bone marrow interfere with biosynthesis of heme
-caused by genetic inheritance of drugs/ bone marrow toxins (lead, antibiotics, chemotherapeutics)
-ringed sideroblasts are highly indicative of the disease
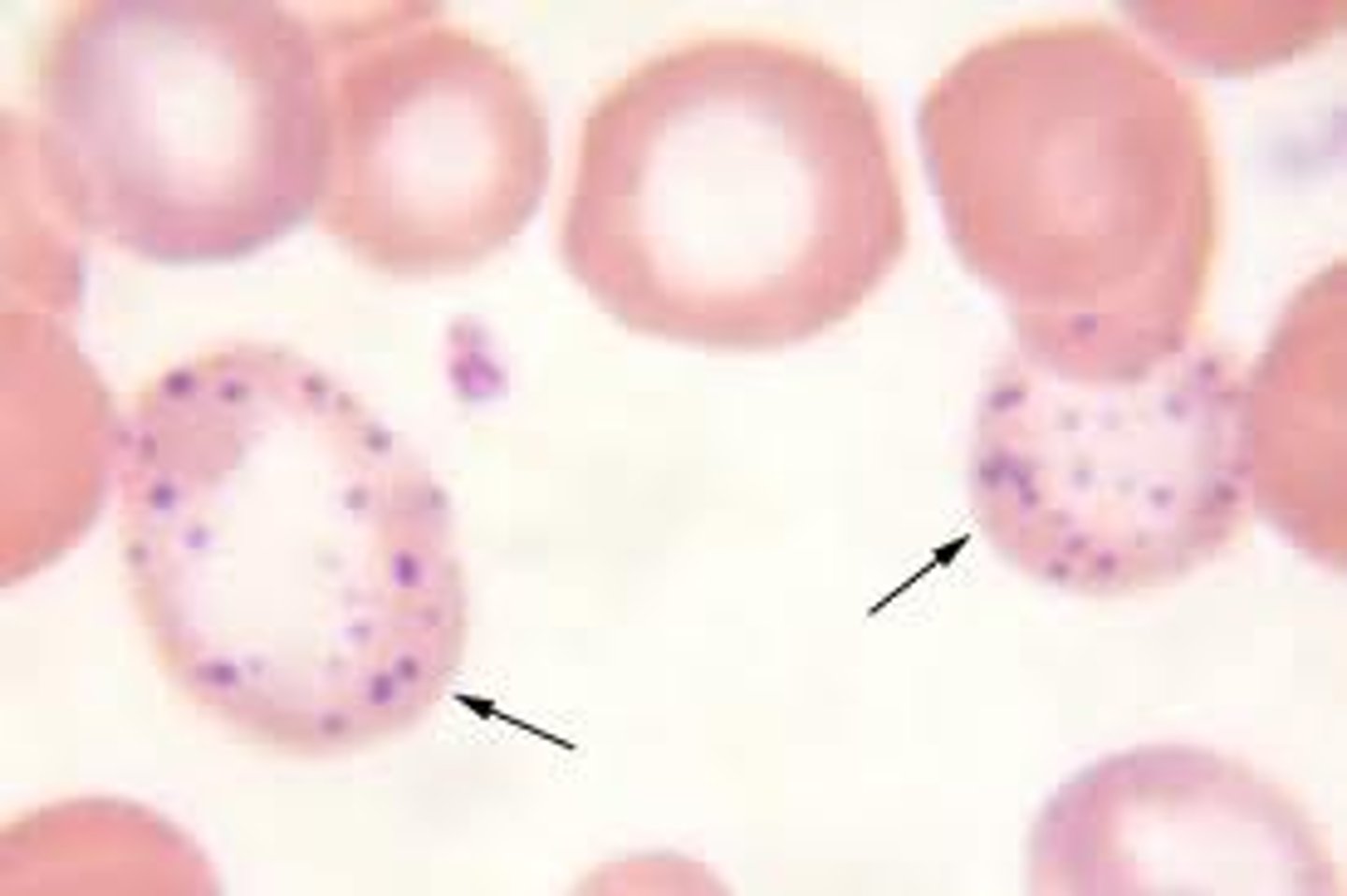
-basophillic stippling is common in lead poisoning
-normocytic normochromic cells
iron deficiency anemia (IDA)
-caused by inadequate intake, increased need or malabsorption of iron, poor diet or chronic blood loss
-symptoms: fatigue, weakness, pallor, spooning of the nails (koilonychia) and pica
-H/H decreased
-microcytic, hypochromic cells
-marked poikilocytosis (target cells, spherocytes, tear drop cells and schistocytes)
-FEP, sTR and TIBC increased
-ferritin, hepcidin and serum iron decreased
anemia of chronic inflammation
-anemia occurring secondary to underlying condition (chronic inflammatory disease, chronic infection or malignancy) that causes release of cell products
-hepcidin, lactoferrin and inflammatory cytokines cause decreased iron status and anemia
-low Hgb
-low TIBC (hepcidin is increased due to acute phase reaction)
-normocytic normochromic anemia
-serum iron decreased
-ferritin (acute phase reactant) and FEP increased
hereditary hemochromatosis (HH)
-inheritance of mutated HFE gene inhibits production of hepcidin, leading to constant activation of ferroportin
-increased levels of iron in circulation are exposed to oxygen and produce damaging superoxide ions
-symptoms: begin between 30-40, iron deposits on organs (pancreas), bronzed diabetes, cell death, release of lysosomal enzymes
-increased serum ferritin and transferrin saturation
-genetic testing reveals mutated HFE gene
hereditary hemochromatosis treatment
-therapeutic phlebotomy: 500 mL of blood is removed per week to decrease serum iron
megaloblastic anemia
-impaired DNA synthesis due to deficiency of Vitamin B12 and/ or folate leads to decreased number of cell divisions
-produces large macrocytes with immature nuclei
-symptoms: fever, glossitis, loss of appetite, neurologic abnormalities (pins and needles, numbness, hallucinations and paranoia/ megaloblastic madness)
-pancytopenia
-decreased H/H
-macrocytosis
-increased MCV, high RDW
-hypersegmented neutrophils
-nuclear cytoplasmic asynchrony
_M:E ratio 1:1 to 1:3
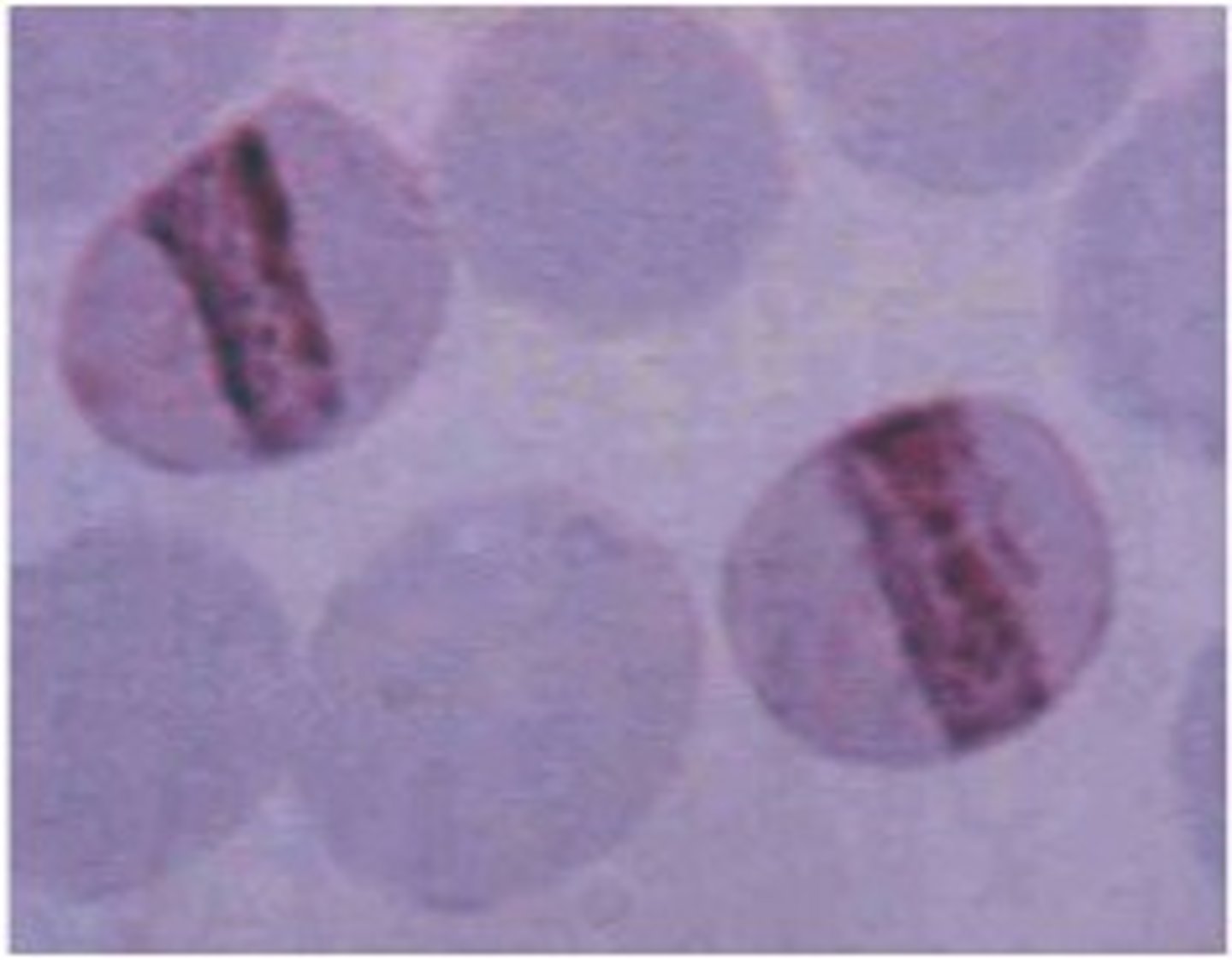
-teardrop cells, schistocytes and microspherocytes in PB
-Howell jolly bodies (DNA remnants) and cabot rings (figure 8)
-increased bilirubin and LDH
G6PD deficiency
-decrase in G6PD enzyme causes underproduction of NADPH, leading to inability to reduce glutathione for detox of H2O2
-H2O2 oxidizes hemoglobin in the RBCs, leading to formation of heinz bodies
-can be induced by oxidative drugs
-symptoms: jaundice, anemia, hyperbilirubinemia
-N/N anemia
-anisocytosis, poikilocytosis
-spherocytes and schistocytes in PB
-heinz bodies seen with crystal violet
-G6PD activity decreased
-fluorescent spot test negative
hereditary spherocytosis (HS)
-proteins in the RBC membrane disrupt vertical interactions and destabilize the lipid bilayer, causing loss of membrane material that leads to formation of spherocytes
-mutations in genes for ankyrin, alpha and beta spectrin or protein 4.2 lead to increased membrane permeability to Na+ and K+, leading to cellular dehydration
-splenic conditioning: abnormal RBCs are targeted by macrophages and lead to anemia
-HS triad: N/N anemia, jaundice and splenomegaly
-spherocytes and polychromasia in PB
-increased MCHC and RDW
-increased osmotic fragility
-EMA binding test: low fluorescence
-autohemolysis test: 10-50%, decreased in presence of glucose
hereditary spherocytosis (HS) treatment
-splenectomy: prevents targeting of RBCs by splenic macrophages and keeps cells in circulation longer
babesia
-tick transmitted disease
-can be transmitted by transfusion of infected blood unit
-symptoms: can be asymptomatic, fever, chills, headache, sweats, nausea, fatigue, jaundice, splenomegaly, hepatomegaly
-H/H decreased
-reticulocytosis
-decreased serum haptoglobin
-bilirubinemia
-leukopenia, thrombocytopenia
-hemoglobinuria, proteinuria
50-160 (ug/dL)
serum iron reference range
20-55(%)
percent transferrin saturation reference range
250-400 (ug/dL)
TIBC reference range
40-400 (ng/mL)
male serum ferritin reference range
12-160 (ng/mL)
female serum ferritin reference range
< 40 (ug/dL)
FEP reference range
glossitis
sore, inflamed tongue
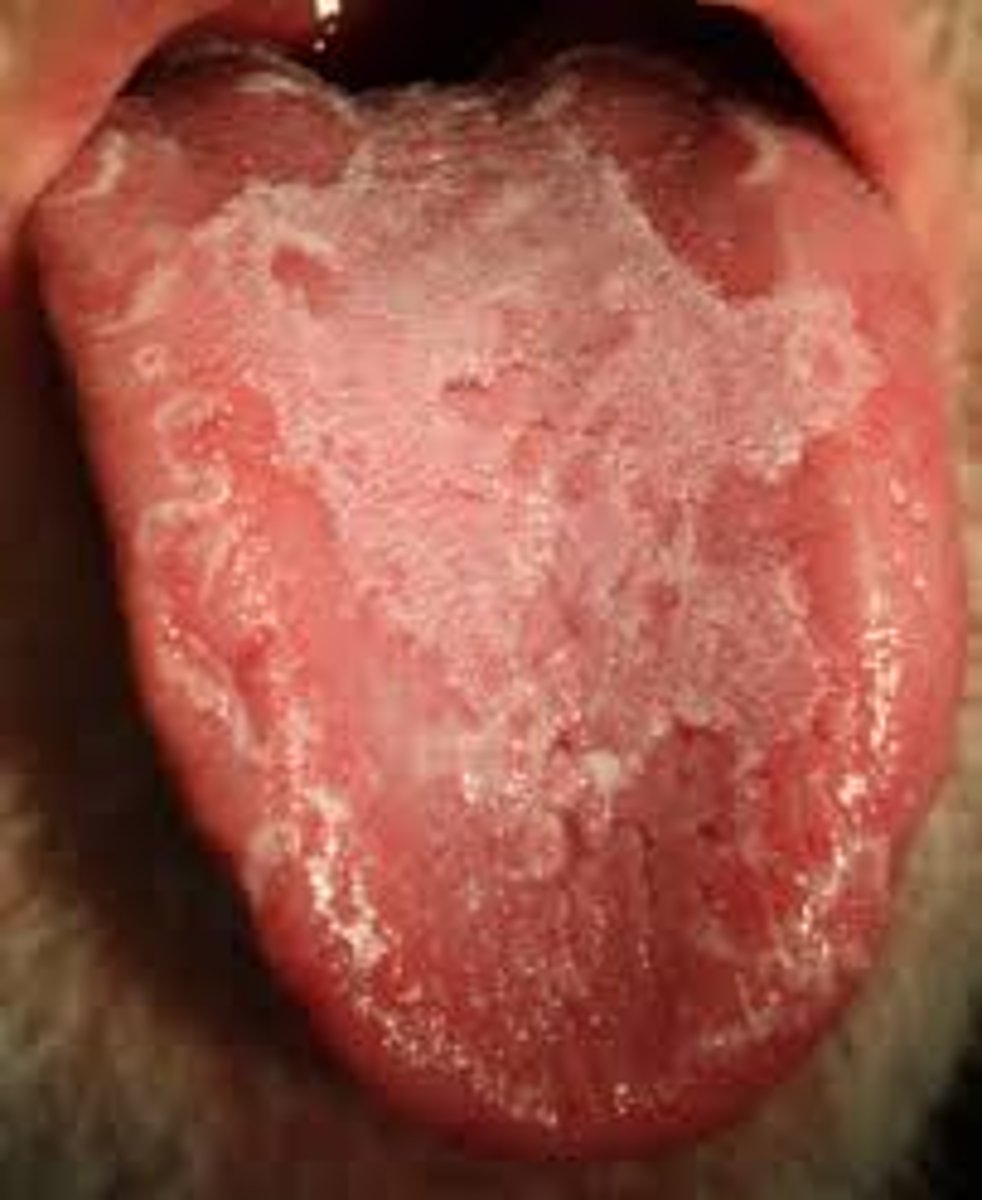
pica
craving for nonfood items (e.g. clay or dirt)
iron deficiency anemia treatment
-treat underlying cause of poor iron status (correct diet, bleeding, malabsorption, etc.)
-oral or parenteral supplemental iron
-BRC transfusion (extreme cases only)
cytokines in anemia of chronic inflammation
-hepcidin: inflammation causes release of hepcidin as acute phase reactant (not due to adequate iron levels), inactivating ferroportin and inhibiting iron release into circulation
-lactoferrin: activated neutrophils release lactoferrin to compete with bacteria for iron, lactoferrin will out-compete body cells for iron
-cytokines: TNF alpha, INF gamma, IL1, etc. interfere with ferrokinetics and erythropoiesis, leading to anemia
anemia of chronic inflammation treatment
-erythropoietin (EPO) hormone treatment
-iron supplements
lead poisoning (effects)
leads to alteration in heme biosynthesis
-interferes with ALA dehydratase, leading to buildup of ALA that is released into urine
-interferes with incorporation of ferrous iron into protoporphyrin IX
lead poisoning treatment
-administer EDTA to chelate lead (released in urine)
ringed sideroblasts
iron congregates in the mitochondria of developing erythroblasts
-*hallmark of sideroblastic anemia)
basophilic stippling
ribosomal RNA in reticulocytes breaks down
-hallmark of lead poisoning
erythropoietic protoporphyrias (EPP)
-mutated FECH gene leads to lack of ferrocheletase enzyme which impairs production of porphyrins
-symptoms: photosensitivity, anemia
-propoporphyrin increased in RBCs and in feces
-ferrochelatase enzyme decreased or missing
-genetic testing reveals mutated FECH gene
porphyria
impaired production of porphyrin component of heme due to missing or deficient enzymes
-leads to buildup of products from pathway in the tissues
-cell death causes products to leak into urine and feces
-severe burns when exposed to sunlight due to fluorescent products
schillings test
test that was once used to diagnose pernicious anemia
-part 1: oral intake of radiolabeled B12 and IM dose of nonlabeled B12, 24 hour urine sample is assesed for absorption of vitamins (>5%= normal, <5%=impaired absorption)
-part 2: if part 1 is impaired, test is repeated with addition of intrinsic factor to oral dose (>5%= pernicious anemia due to lack of IF, <5%= malabsorptive disorder)
disorders of ineffective erythropoiesis
-megaloblsatic anemia
-aplastic anemia
pernicious anemia (PA)
-autoimmune destruction of parietal cells by CD4+ T cells leads to lack of intrinsic factor, which inhibits B12 absorption
-B12 deficiency inhibits DNA synthesis, leading to anemia and megaloblastic RBCs
-symptoms: usually appear in 6th decade of life, fever, glossitis, lack of appetite, neurologic abnormalities (pins and needles, numbness, hallucinations, paranoia/ megaloblastic maddness)
-serum B12 decreased
-achlorhydria: lack of H+ in stomach
-increased gastrin
-decreased H/H
-*blocking antibodies to IF and parietal cells
-macrocytosis
-pancytopenia
-hypersegmented neutrophils
pernicious anemia treatment
-intramuscular injection of B12 (bypasses need for IF to absorb)
vitamin B12 (cobalamin)
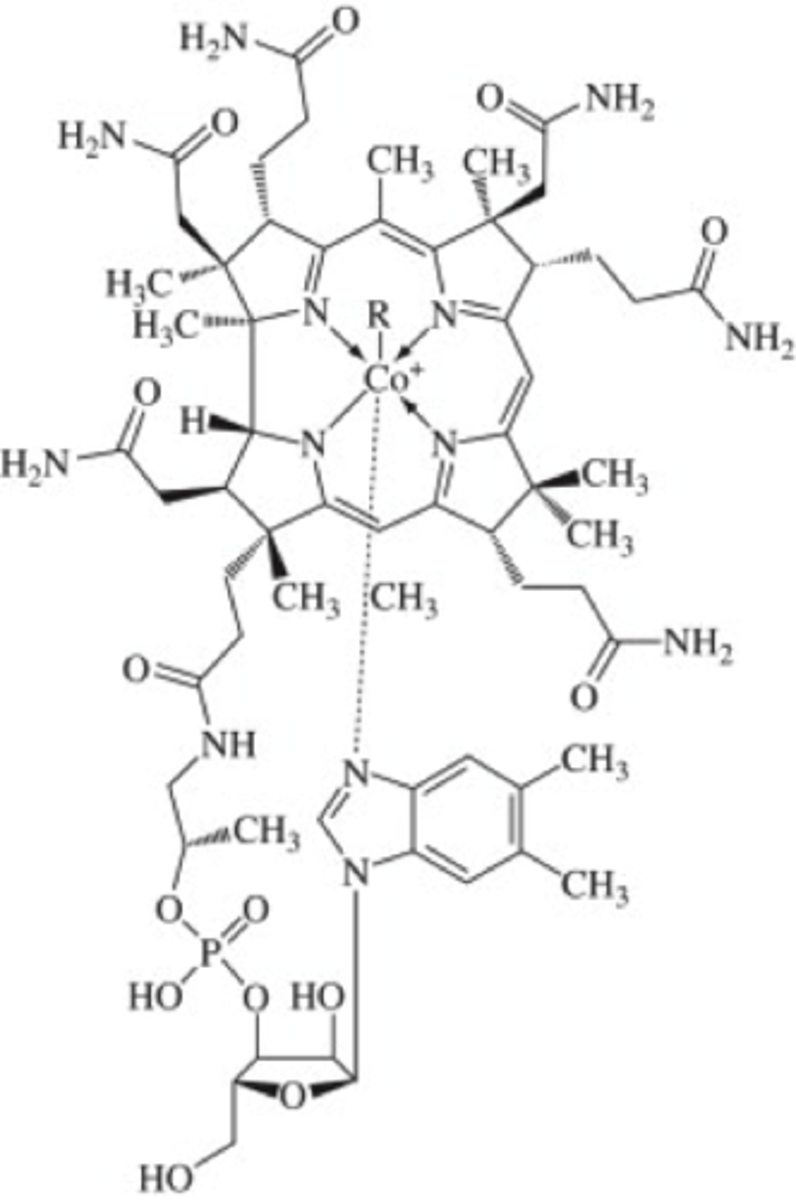
folate
many different forms
diphyllobothrium latum (fish tapeworm)
-parasite that is capable of splitting cobalamin from IF and makes the cobalamin unable to absorb
-leads to deficiency
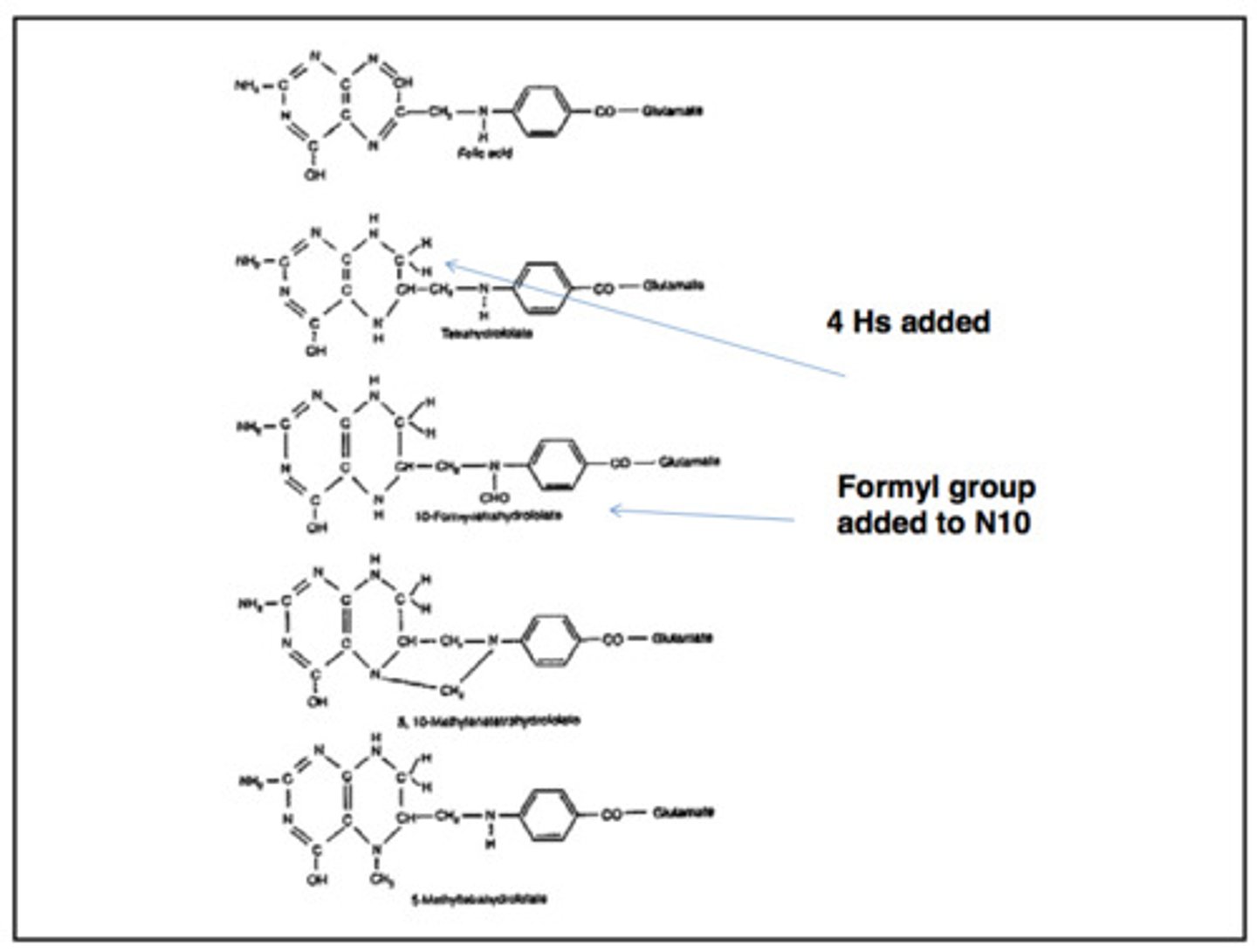
folate absorption
-folate polyglutamate is taken in from the diet, and converted to folate monoglutamate
-folate monoglutamate is taken up into enterocytes and converted into 5-methyl THF
- 5-methyl HF is released into circulation and is taken up by cells to catalyze reactions needed for DNA synthesis
vitamin B12 (cobalamin) absorption
-pepsin and HCL remove B12 from food products, B12 binds to haptochorrin
-B12 is cleaved from haptochorrin by pancreatic protases
-parietal cells release intrinsic factor, which binds to B12 and allows it to be taken up into enterocytes via cuban receptors
-IF and B12 are separated in the enterocyte, and B12 binds to transcobalamin to be transported through portal circulation
-B12 is used as a coenzyme in DNA synthesis
methylmalonic acid
-in the absence of vitamin B12, the activity of methyl malonyl CoA reductase is inhibited
-high serum levels of methylmalonic acids build up
-common in B12 deficiencies (i.e. pernicious anemia)
DNA synthesis
-methionine synthase and its vitamin B12 cofactor transfer a methyl group from 5-methyl THF to homocysteine, generating methionine and THF
-THF is converted to 5,10- methelene THF by gaining methyl groups from serine
- 5,10-methylene THF is converted to dUMP
-dUMP is converted to dTMP
-dTMP is converted to dTTP, which is used in DNA synthesis
folate trap
if vitamin B12 is not available as a cofactor for the methionine synthase, folate is metabolically trapped as 5-methyl THF and DNA synthesis cannot proceed
aplastic anemia lab findings
-symptoms: insidious onset, pallor, fatigue, tachycardia, hypotension, cardiac failure, bleeding (petechiae, ecchymoses), fever-neutropenia
-pancytopenia
-low Hgb
-retics decreased
-serum iron and % transferrin saturation increased
-neutropenia
-increased adipocytes in bone marrow, severe hypocellularity of RBC precurors
fanconi's anemia (FA) etiology
-congenital form of aplastic anemia due to inherited bone marrow failure
-inheritance of FANCA or FANCB gene leads to chromosome instability and increased breakage when exposed to DNA cross linking agents
fanconi's anemia (FA) symptoms
-physical malformations present at birth in 2/3 of affected individuals
-radial hypoplasia, microencephaly, hip dislocation, hyper/ hypopigmentation, short stature, low birth weight, triphalangeal thumb
-bone marrow failure by age 40
-increased risk of cancers/ tumors
fanconi's anemia (FA) lab findings
-chromosome instability testing: increased breakage when exposed to DNA cross-linked agents (diepoxybutane or mitomycin C)
-lymphocytes have increased chromosomal fragility
-pancytopenia
-reticulocytopenia
-hypocellular BM
-macrocytic RBCs (increased MCV)
Fanconi's anemia (FA) treatment
hematopoietic stem cell transplant in BM
pancytopenia
a decreased in all cell lines (WBC, RBC and PLT)
-can be caused by deficiency of folate/ B12
G6PD class I
<1% activity of G6PD
G6PD class II
<10% activity of G6PD
-can be induced by fava beans
G6PD class III
10-60% activity of G6PD
G6PD class IV
60-150% activity of G6PD
-no clinical symptoms
G6PD class V
>150% G6PD activity
-no clinical symptoms
G6PD deficiency lab findings
-N/N anemia
-decreased H/H
-anisocytosis/ poikilocytosis
-spherocytes, schistocytes and heinz bodies present in PB
-increased retics, polychromasia
-serum haptoglobin, LDH and indirect bili increased (hemolysis)
-fluorescent spot test negative
PK deficiency lab findings
-reticulocytosis
-anisocytosis, poikilocytosis
-increased bili and LDH
-decreased haptoglobin
-normal osmotic fragility
-autohemolysis test does not correct itself in presence of glucose
-fluorescent spot test increased
autohemolysis test (HS vs. PK deficiency)
-hereditary spherocytosis: 10-50% hemolysis, corrected by adding glucose
-PK deficiency: adding glucose to system does NOT decrease hemolysis
PNH pathophysiology
-acquired stem cell mutation that results in lack of GPI anchor proteins, so CD55 and CD59 are unable to stay on the surface of cells
-lack of CD55 and CD59 regulatory proteins prevents shutting off complement cascade, MAC continues lysing cells
-RBCs are lysed leading to intravascular hemolysis
-hemoglobinuria is most pronounced in the mornings
malaria transmission
sporozoites in the salivary glands of infected mosquitoes are transferred to human during blood meal
babesia transmission
sporozoites in salivary glands of a tick are transmitted to human during blood meal
-transfusion with RBCs from an asymptomatic host also possible
thick smear
large drop of blood that is wright stained and fixed with methyl alcohol to visualize if plasmodium parasites are present
-cannot identify species
thin smear
blood is spread out on a slide to created a feathered edge, allowing identification of plasmodium species and count of % parasitemia
plasmodium falciparum
infects RBCs of all ages, leading to high level of parasitemia
-gametocytes cause crescent shaped RBC
-most severe form of malaria, leads to cerebral malaria
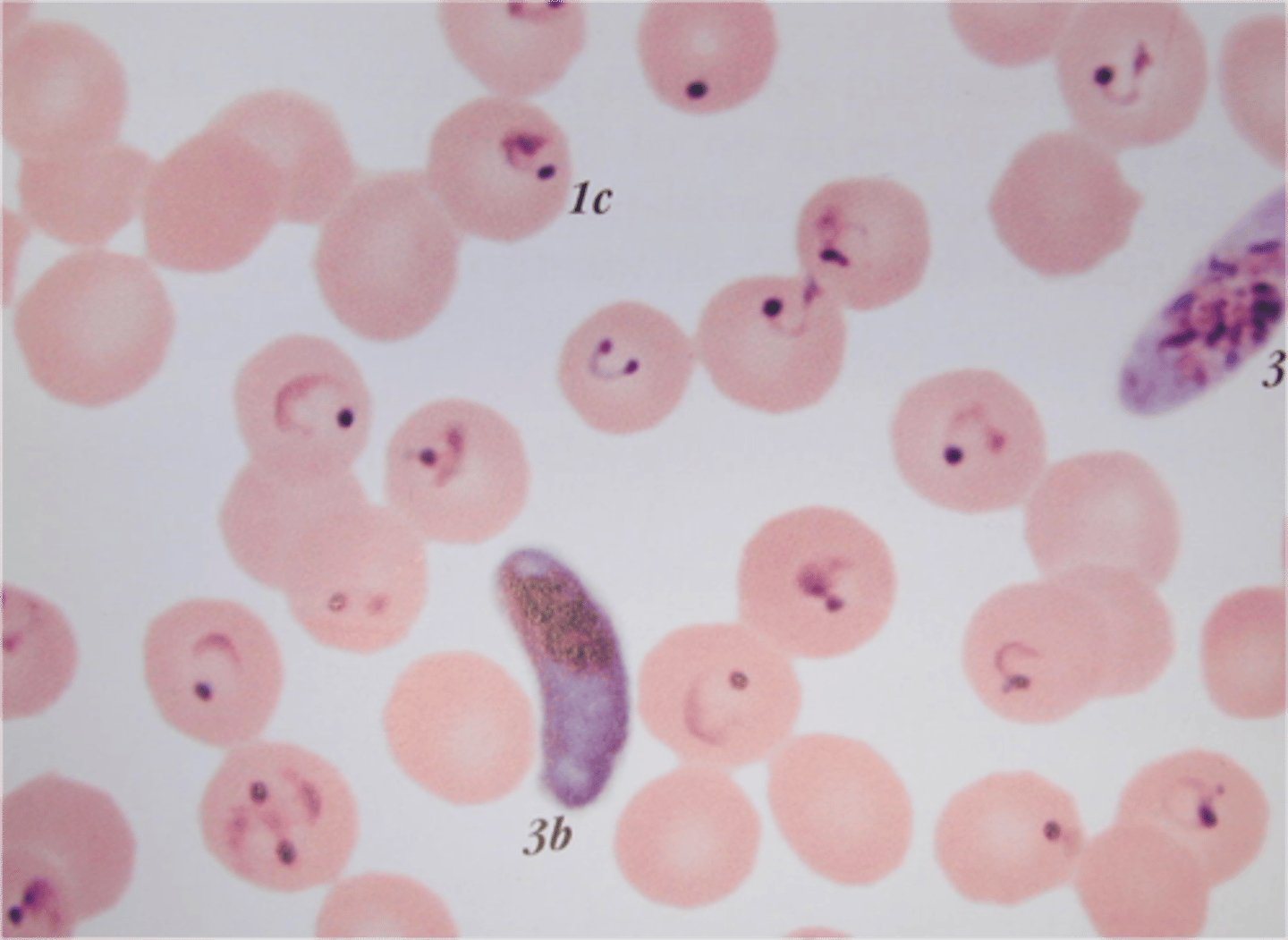
plasmodium knowlessi
infects RBCs of all ages
-ring forms can have multiple rings in same RBC
-trophozoites visible on thin smears
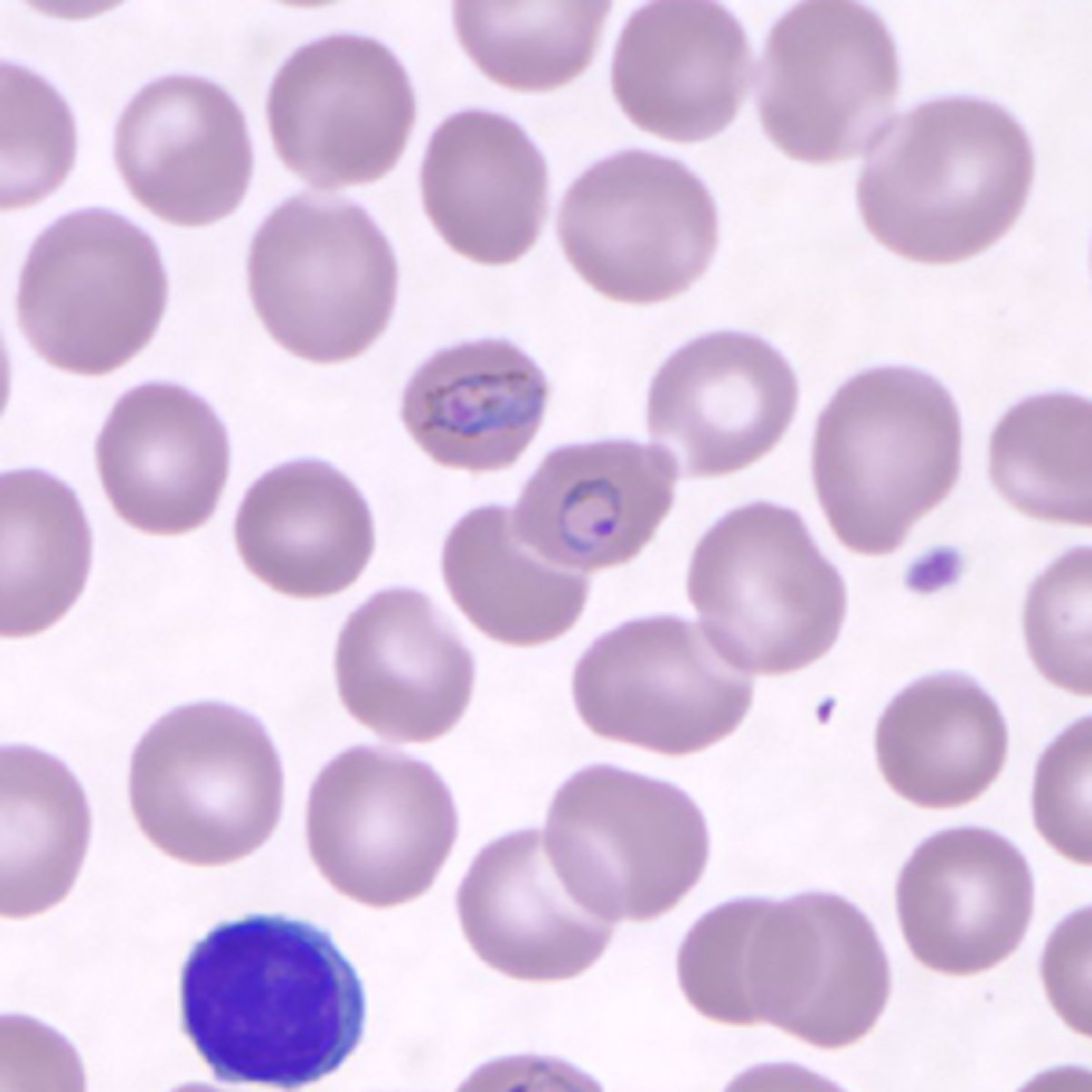
plasmodium vivax
infects reticulocytes with duffy antigens present
-trophozoites cause ring forms in RBCs
-infected RBCs appear enlarged
-schuffner's stippling and ameboid appearance in infected RBCs
-schizonts visible in thin smears
-merezoites cause formation of brown hemozoin pigment
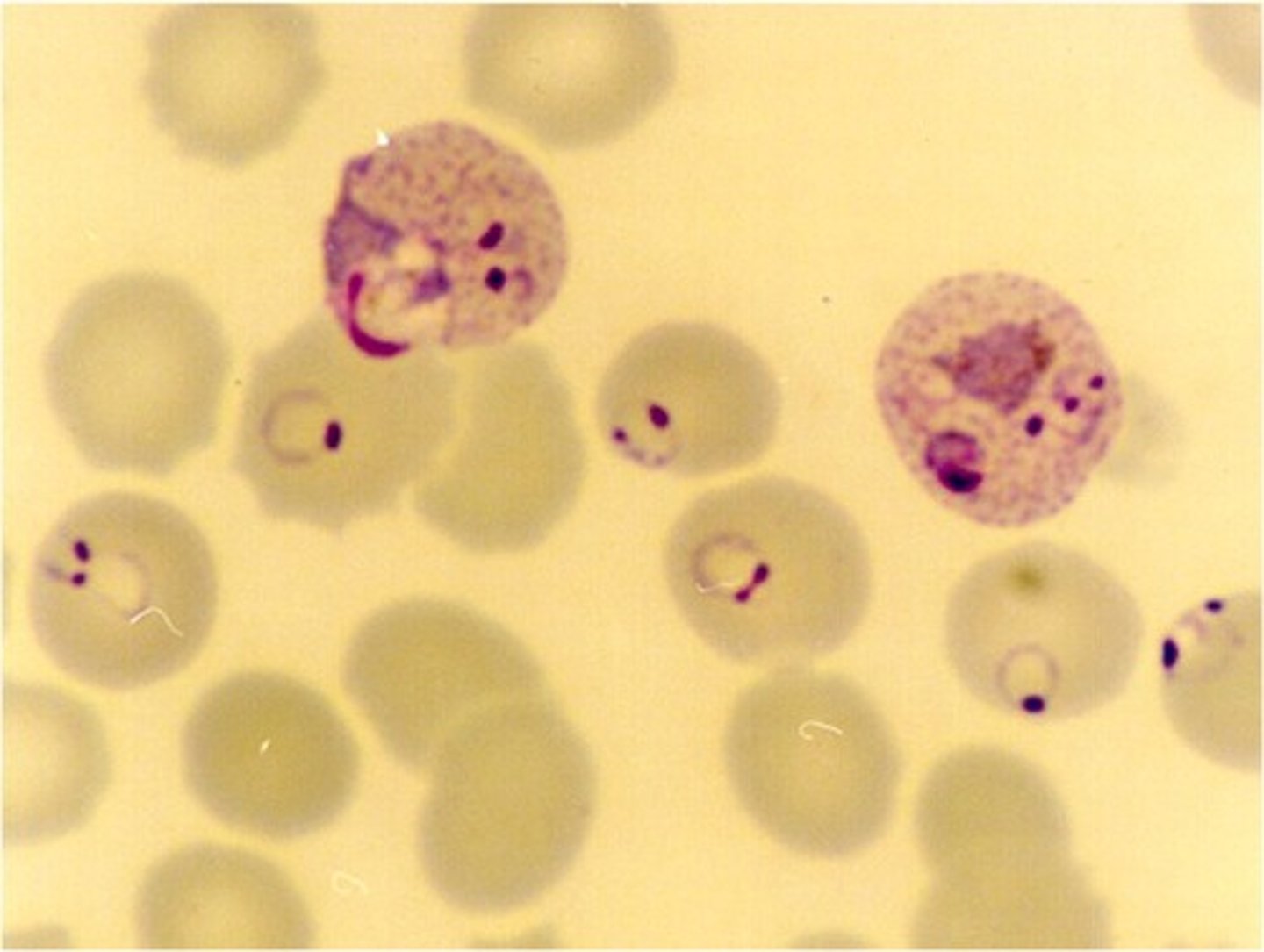
plasmodium ovale
infects reticulocytes
-infected cells appear oval shaped with fringed edges
-Schuffner's slipping present in trophozoite stage
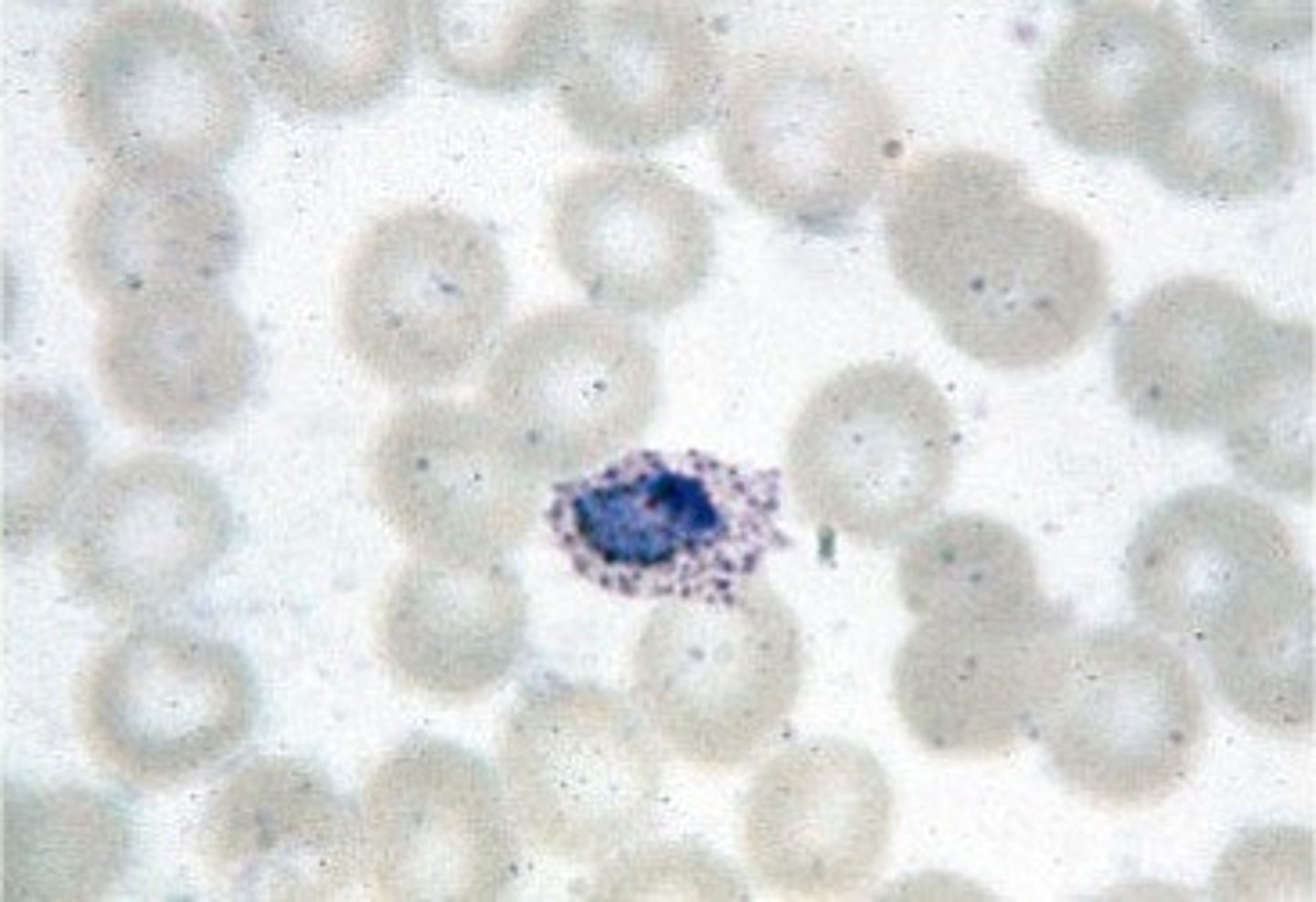
plasmodium malariae
infects older RBCs
-band form causes a thin, dark band to form across the RBC
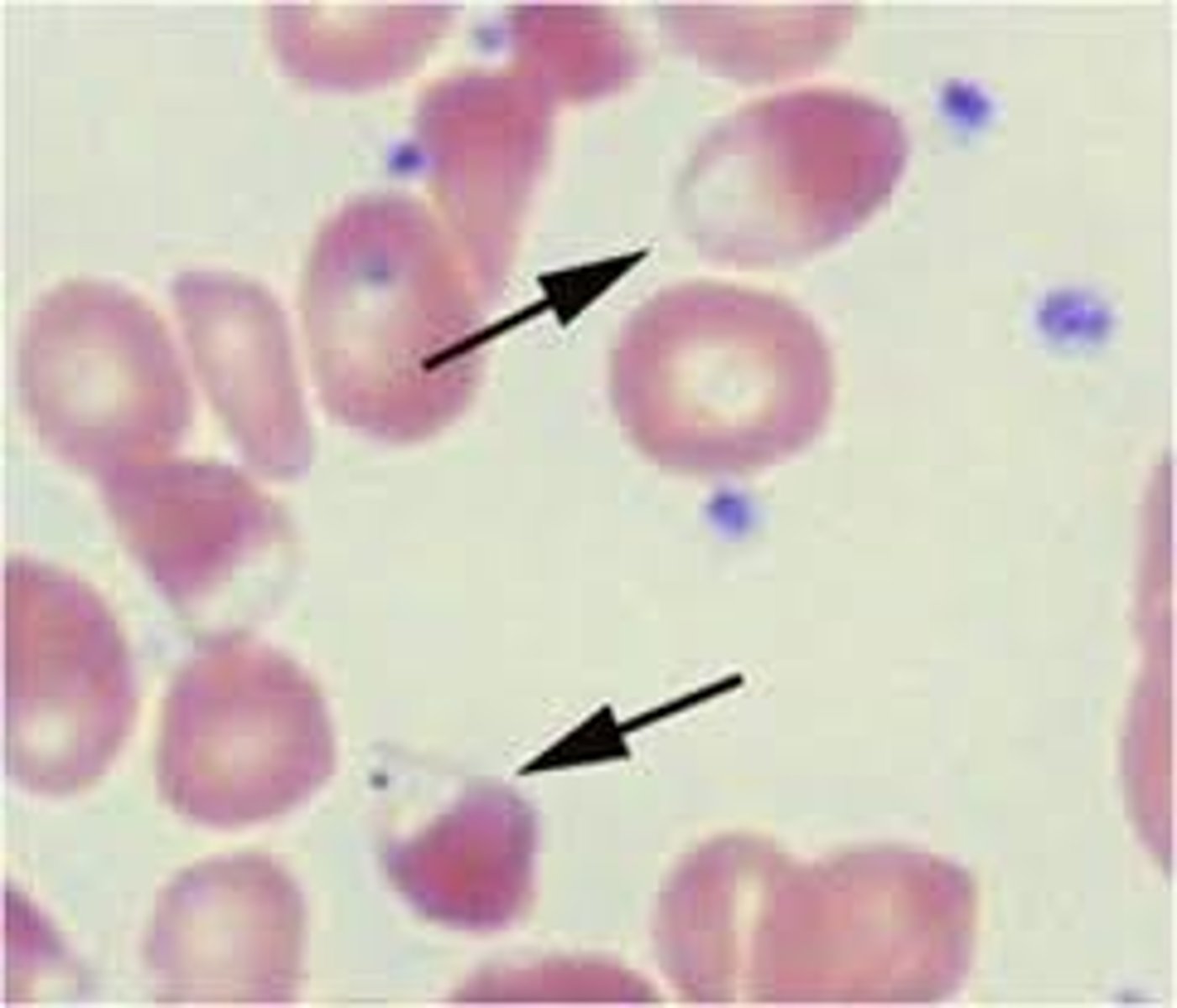
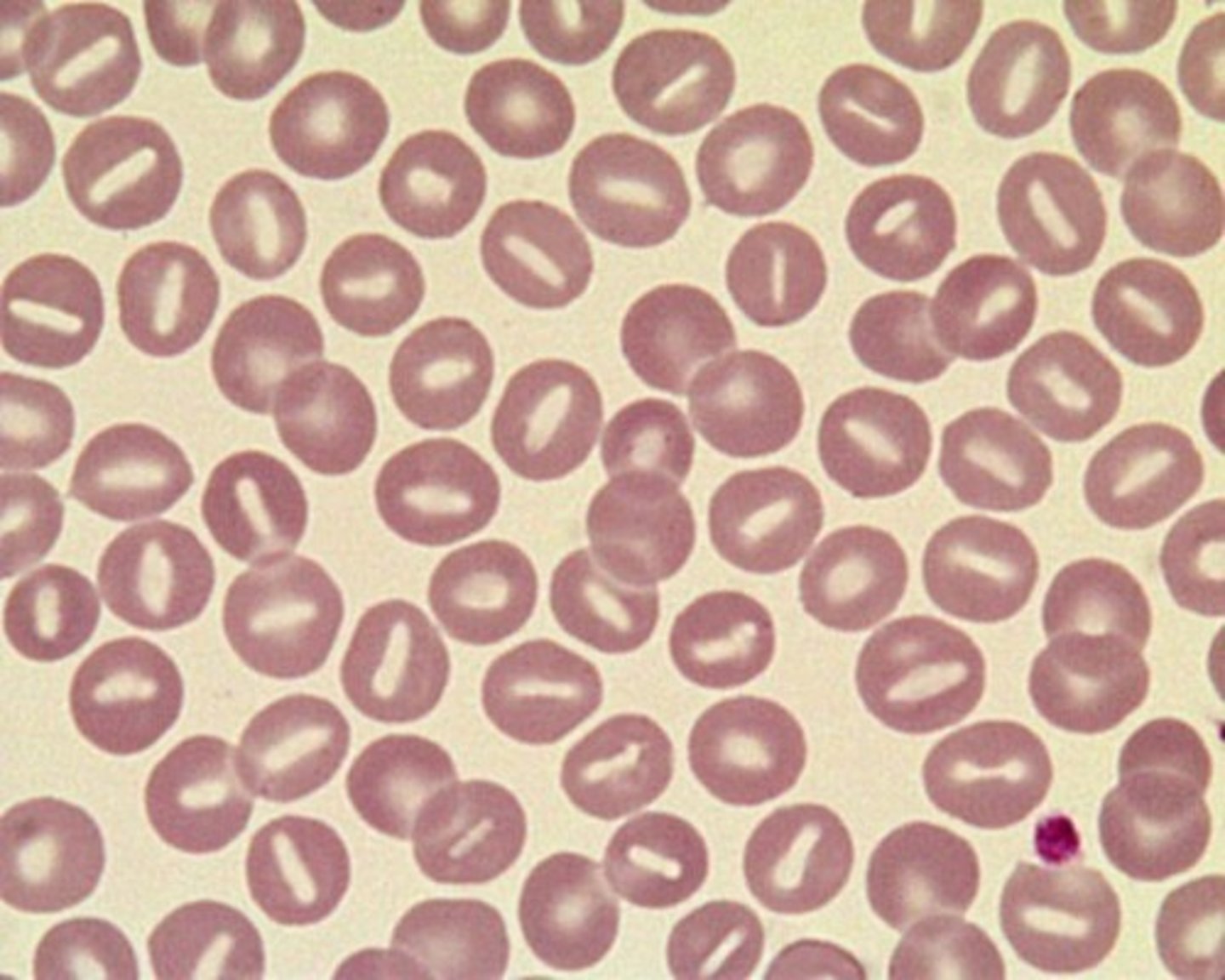
hereditary xerocytosis (HX)
-inherited mutation of the PIEZ01 gene leads to defects in membrane permeability that allows K+ to leak out of the cell, leading to dehydrated RBCs
-symptoms: mild to moderate anemia, hydrops fetalis, jaundice and splenomegaly
-reticulocytosis
-increased MCHC
-decreased osmotic fragility
-stomatocytes, target cells, burr cells
-puddled hemoglobin (shown in picture)
hereditary xerocytosis (HX) treatment
-usually not necessary
-supportive transfusions
hereditary pyropoikilocytosis (HPP)
-a variant of HE that causes extreme poikilocytosis with a large number of schistocytes
-EMA binding test: low fluorescence
-increased thermal sensitivity (fragmentation at 41-45 C)
-low MCV
hereditary pyropoikilocytosis (HPP) treatment
-splenectomy and supportive transfusions
hereditary elliptocytosis (HE)
-inherited hemolytic anemia caused by defective alpha / beta spectrin, or protein 4.1 that disrupts horizontal interactions in the RBC cytoskeleton
-mutations in SPTA1, SPTB and EP41 genes disrupt interactions of the spectrin dimer and destabilize the cytoskeleton, forming elliptocytes
-symptoms: usually asymptomatic, mild compensated anemia
-cigar shaped elliptocytes
-increased osmotic fragility
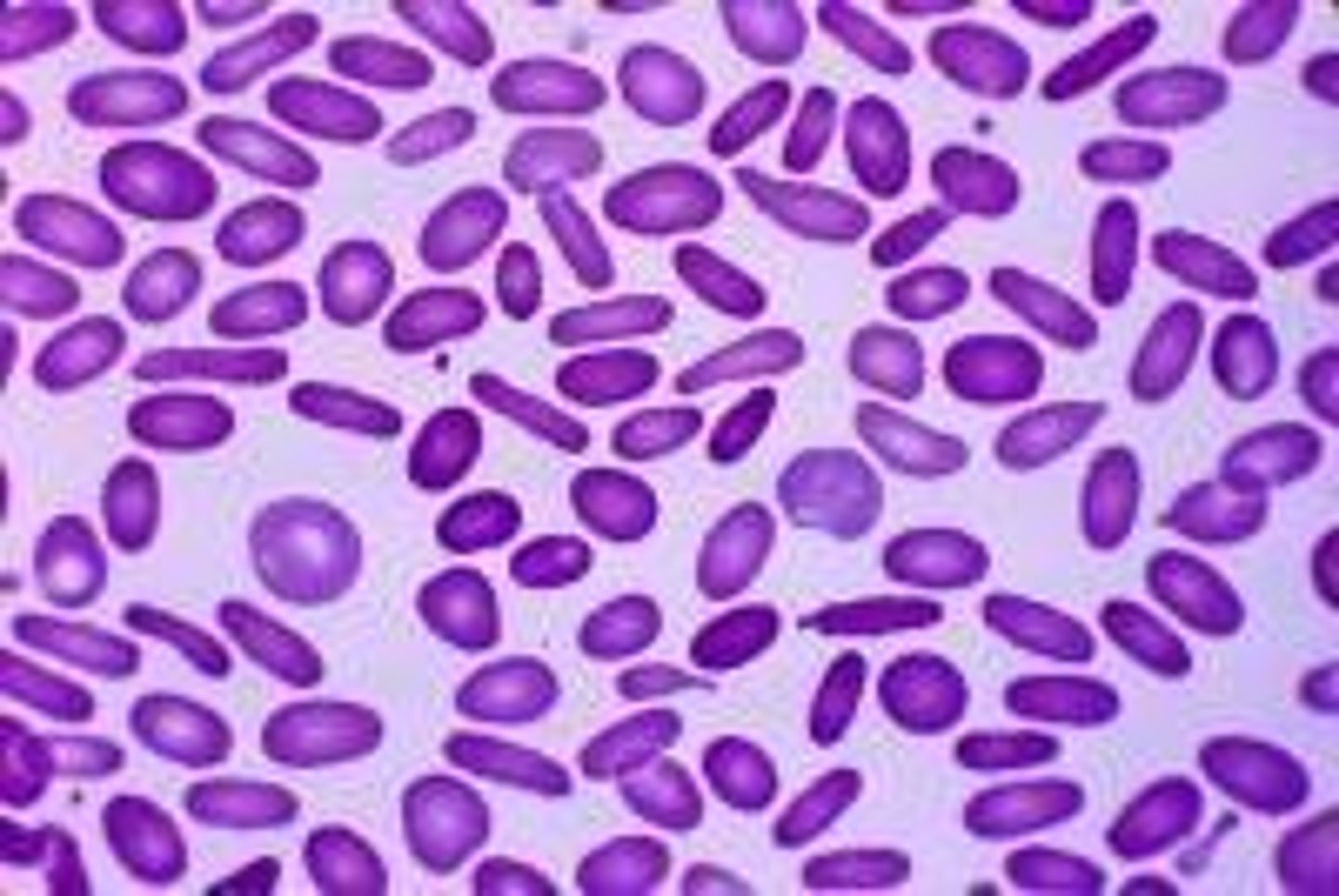
hereditary elliptocytosis (HE) treatment
-splenectomy
-RBC transfusions to treat anemia
south asian ovalocytosis (SAO)
-a variant of HE caused by inherited mutation to SLC4A1 gene, producing a mutated Band 3 protein
-cells have increased rigidity due to mutated band 3 protein
-provides resistance to P. malaria parasite (increased incidence of mutation in malaria endemic parts of the world)
-ovalocytes with 1-2 transverse ridges seen in PB
-no treatment needed
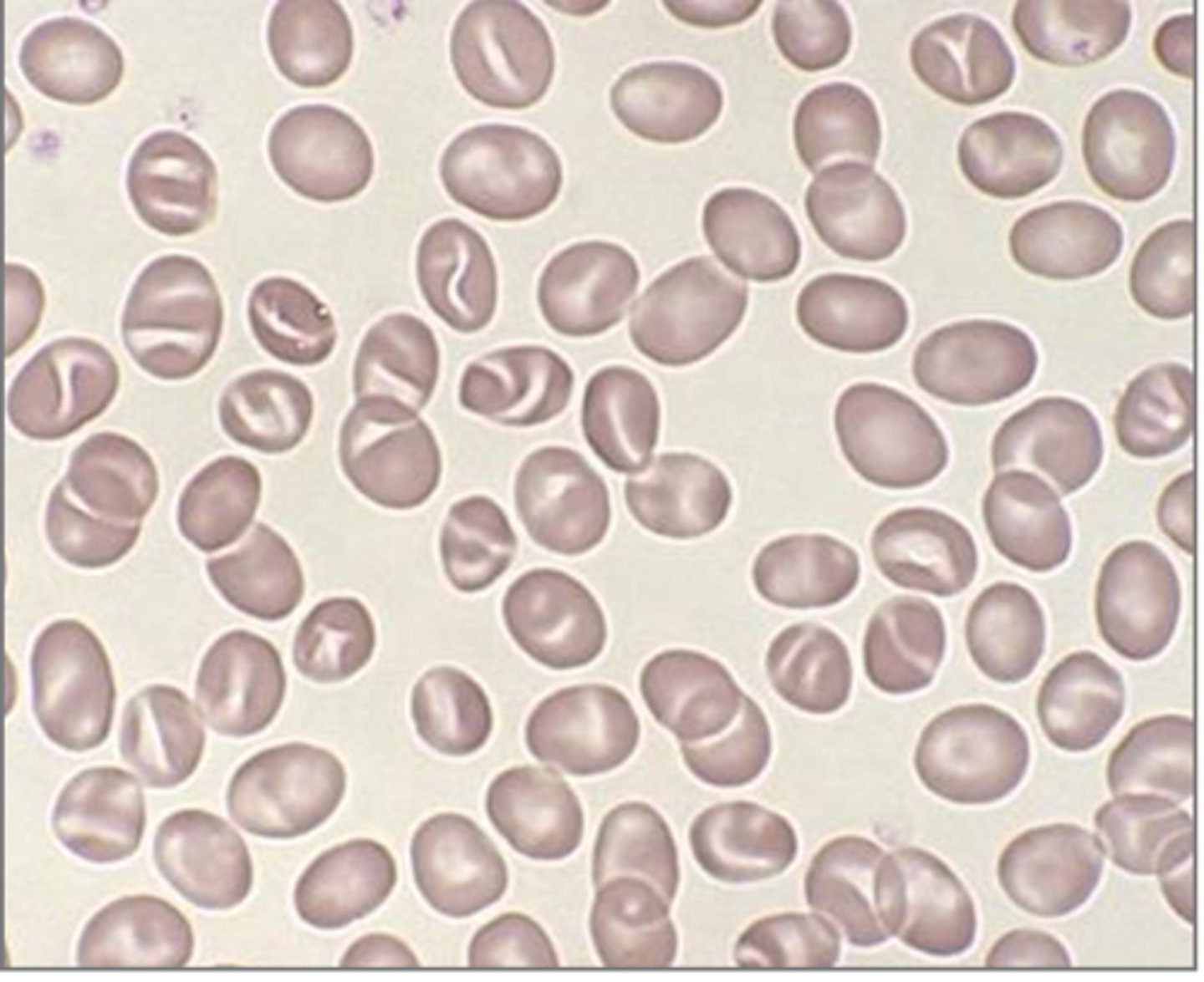
stomatocytes
RBC morphology associated with Rh null syndrome
In this disorder, target cells, stomatocytes, burr cells are present, "puddled" hemoglobin, and the MCHC is increased with a decreased osmotic fragility.
A. Hereditary Elliptocytosis
B. Southeastern Asian Ovalocytosis
C. Hereditary Spherocytosis
D. Hereditary Xerocytosis
hereditary xerocytosis
All of the following are true regarding Erythropoietic Protoporphyria, except:
A. ferrocheletase is deficient
B. patients exhibit photosensitivity
C. inheritance is autosomal dominant
D. enzyme deficiency is Uroporphyrinogen III synthase
enzyme deficiency is uroporphyrinogen III synthase
All of the following is true regarding folate deficiency, except:
A. Hgb is decreased
B. white blood cells are increased
C. RBC folate is decreased
D. neutrophils are hypersegmented
white blood cells are increased
All of the following is true regarding Stage 1 of iron deficiency, except:
A. serum ferritin levels are normal
B. serum ferritin levels are low
C. patients are devoid of symptoms of anemia
D. RBC development is normal
serum ferritin levels are normal
The test that is most useful in differentiating Fanconi's Anemia from other causes of pancytopenia is:
A. bone marrow biopsy
B. flow cytometric analysis of CD55
C. Ham acidified serum test
D. Diepoxybutane-induced chromosome breakage
diepoxybutane-induced chromosome breakage
All of the following are associated with sideroblastic anemia except:
A. increased red cell protoporphyrin
B. dimorphic blood picture
C. ringed sideroblasts
D. increased serum iron
increased red cell protoporphyrin
Plasmodium ovale typically invades only:
A. prorubricytes
B. reticulocytes
C. metarubricytes
D. rubriblasts
reticulocytes
Which of the following stains blue with Prussian blue stain?
A. apoferritin
B. ferritin
C. transferrin
D. hemosiderin
hemosiderin
How are malaria parasites identified in the hematology laboratory?
A. thick and thin peripheral blood smears
B. blood cultures
C. PCR
D. indirect fluorescent test
thick and thin peripheral blood smears
Why is G6PD important for normal red cell survival?
A. alpha chains are produced in excess in absence
B. hemoglobin oxygen affinity is increased in its absence
C. it is required for insertion of iron into the protoporphyrin ring to form heme
D. it is required to regenerate reduced glutathione
it is required to regenerate reduced glutathione
Which of the following are most characteristic of red cell indices associated with megaloblastic anemias?
A. MCV 62 fL, MCH 27 pg, MCHC 30%
B. MCV 78 fL, MCH 23 pg, MCHC 30%
C. MCV 99 fL, MCH 28 pg, MCHC 31%
D. MCV 125 fL, MCH 36 pg, MCHC 34%
MCV 125 fL, MCH 36 pg, MCHC 34%
Which of the following is a typical finding in hereditary spherocytosis?
increased MCHC
What is a hallmark of sideroblastic anemia?
presence of ringed sideroblasts in bone marrow