UPENN NEUR2600: Wk03 Videos
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
What helps form monogamous behaviour in males and females?
Studying voles it was found that monogamous species have high density of oxytocin receptors (females) and vasopressin receptors (males) in certain areas of their brain around hypothalamus.
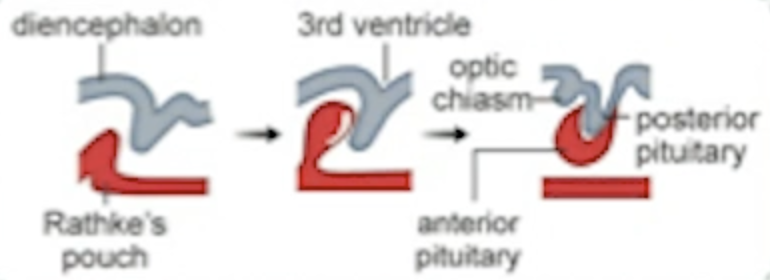
What is Rathke’s pouch?
This embryonic structure originates from the oral ectoderm and contributes to the formation of the anterior pituitary.
AP appears as Rathke’s pouch tissue overlaps the tissue of diencephalon.
What is a homeobox?
A homeobox is a conserved DNA sequence found within genes that regulate the development of body plans and structures in various organisms. It encodes a protein domain, the homeodomain, which binds to DNA and controls the expression of other genes during embryonic development.
For example a mutation in one of the homeoboxes in a fly would make it grow legs on its head instead of antennas.
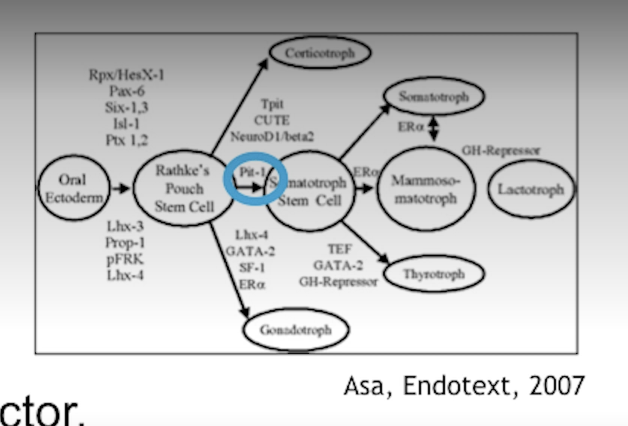
What is pit-1?
A homeobox i.e. a transcription factor in the developing anterior pituitary (first 1-3 months of life) that help AP cells to become somatotrophs.
A mutation in pit-1 means no GH (or even PRL, TSH). Animals without pit-1 become dwarfs.
Lack (or mutation) of which gene in the anterior pituitary will lead to dwarfism?
Pit-1 which is a homeobox for differentiation of AP cells into somatotrophic cells (GH) as well as mammotrophic (PRL) and thyrotropic (TSH).
Chromosome on which the GH gene sits.
Chromosome 17. A huge hormones (190+ amino acids).
Describe GH changes throughout life.
Released episodically, mostly during sleep. Highest level 12-14 years of age.

What’s different about hypothalamic control of GH compared to other hormones?
It has both an accelerator (GHRH) and a break (GHIH aka SST). Both act on G-protein coupled receptors on somatotrophs.
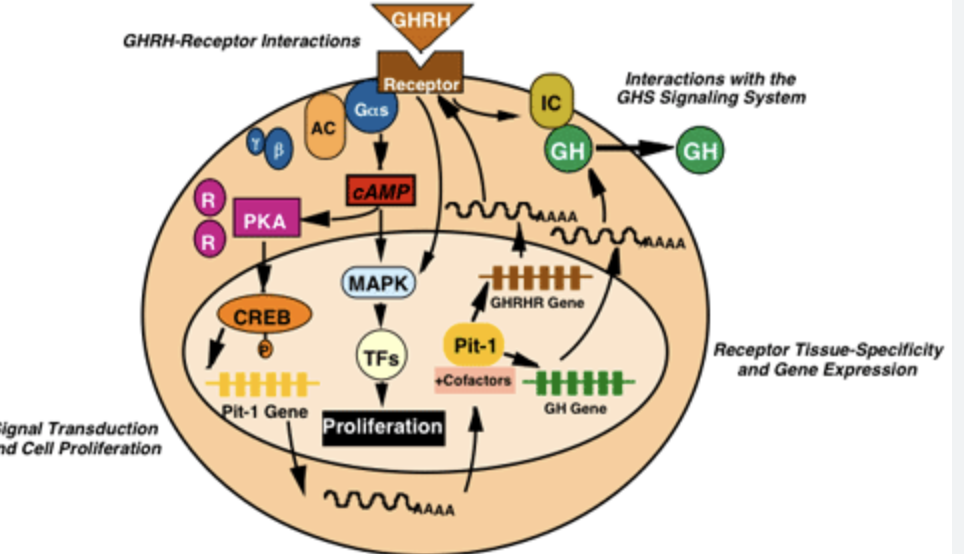
How does GHRH act on somatotrops?
GHRH binds to GHRHR (receptor)
GPCR receptors
Enabled GH release (Ca++ based mechanism)
Increases expression of GH
Increases expression of GHRHR
Increases expression of GH
Increase expression of Pit-1 (homeobox i.e. transcription factor for differentiating cells into somatotropins)
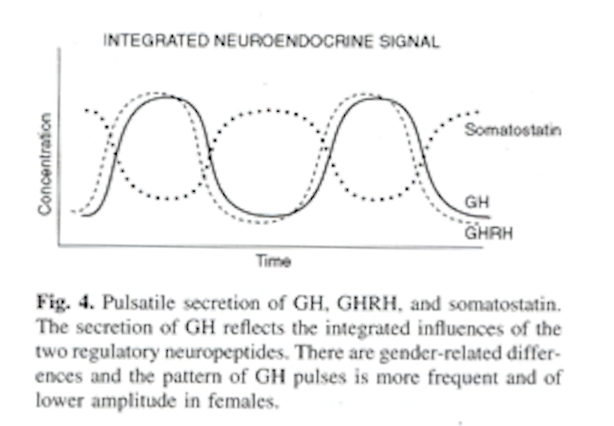
How does GHRH, GH and SST levels change?
GH goes in step with GHRH, SST goes in the opposite direction i.e. when SST is low, GHRH goes up.
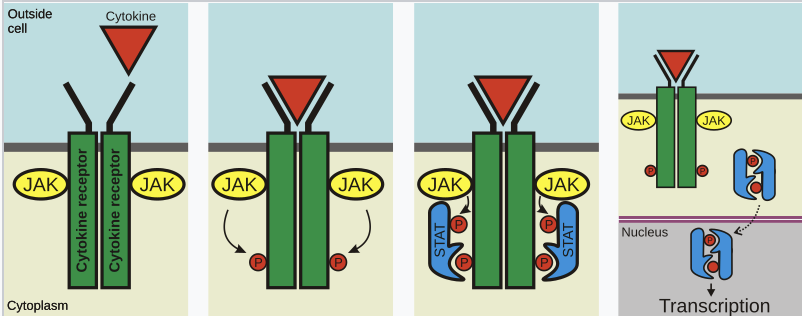
Describe GH receptor.
GH cytokine receptor consists of two units that dimerise when binding GH. They then bind JAK2 which autophosphorylate each other. They then phosphorylate parts of the GHR. This allows STAT proteins to dimerise and translocate into the nucleus.
Where are GH receptors found? What are their role?
Liver - to produce IGF-1
Muscle, bone - growth
Adipose tissue - lipolysis, losing fat
Immune cells - proliferation
Heart - improves heart output
Skin - thickness, wound healing, faster hair growth
Difference between GH and IGF-1 on tissues? Why do we need both?
Both GH and IGF-1 promote growth / anabolism
But IGF-1 has a longer half-life
IGF-1 “amplifies” what GH starts
Tissues respond to GH by producing IGF-1 in a tissue-specific manner (i.e. if tissue needs to grow it will respond to GH with more IGF-1)
IGF-1 can also have paracrine and even autocrine action
So essentially while GH is a “green light” to grow, IGF-1 promotes growth in a more controlled, tissue-specific way.
What’s Laron’s dwarfism?
A particular kind of dwarfism where GH receptor is mutated and the liver produces only low levels of IGF-1.
What do IGF-1 levels look in people who age slower vs those age faster?
Age slower - higher IGF-1 levels
Age faster - lower IGF-1 levels
How do gonadal steroids do to GH?
They stimulate GH and correspondingly IGF-1.
How do gonadal steroids promote and also arrest the growth in terms of height?
Gonadal steroids stimulate GH (and IGF-1) causing rapid body growth (muscle and importantly bones). But they also slowly close epiphysial plates on bones to end growth potential.
List various causes of retarded growth in a child?
mutation in pit-1 (AP never develops somatotrops)
mutation in GH, IGF-1, GHR, GHRH, GHRHR, etc
diabetes (due to lack of insulin)
renal disease
celiac disease (can’t absorb nutrients)
high cortisol (stress .. psychosocial dwarfism => fixable)
high cortisol (tumour)
low thyroid (iodine deficiency)
How do we investigate growth retardation due to GH issues?
measure IGF-1 first
if IGF-1 is low, measure GH (at night)
but also check other hormones - what if the problem is that the pituitary didn’t develop properly
If GH deficiency is found, synthetic GH is available!
Name the two conditions due to GH excess when GH (or GHRH) is excessive when (a) bone plates are closed; (b) bone plates are still not closed.
(a) bone plates are closed => acromegaly (bones can’t grow but other tissues like cartilage grows and they become thick and deformed)
(b) bone plates are not closed => gigantism (they grow, bones become longer, etc)
Acromegaly changes are very gradual, how can one (or their doctor) tell if they have it?
Ask them if they changed their shoe size in the past few years.
How do we treat acromegaly?
tumour => remove if possible
otherwise give SST
sometimes GHRHR mutation causes it to constantly stimulate GH release (even in the absence of GHRH) => need to ablate GH producing cells in the pituitary ..
.. or we could give GHRH antagonist but we don’t have it yet
Would would a mutation in a GHRHR making it constitutively activated lead to when only one copy of that gene is mutated?
It’s a dominant trait, b/c even if one copy of the GHRH receptor gene (e.g. in mom’s DNA or dad’s DNA) is mutated this would mean the somatotropic cells would self-activate and produce GH homrone starting in childhood well before the long bones close. And therefore this would cause gigantism.

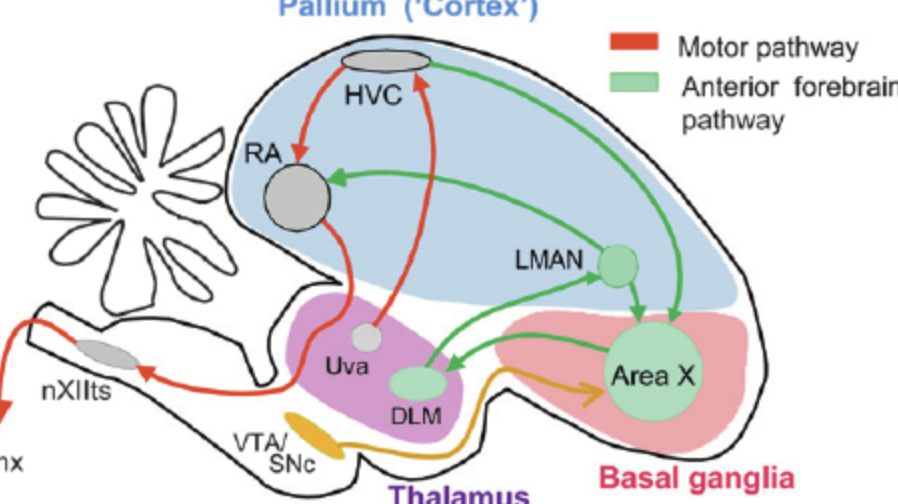
Two pathways controlling bird song.
efferent motor pathway to produce the song (HVC => RA => hypoglossal nucleus => syrinx)
recursive loop i.e. self-feedback (area X => LMAN => RA)
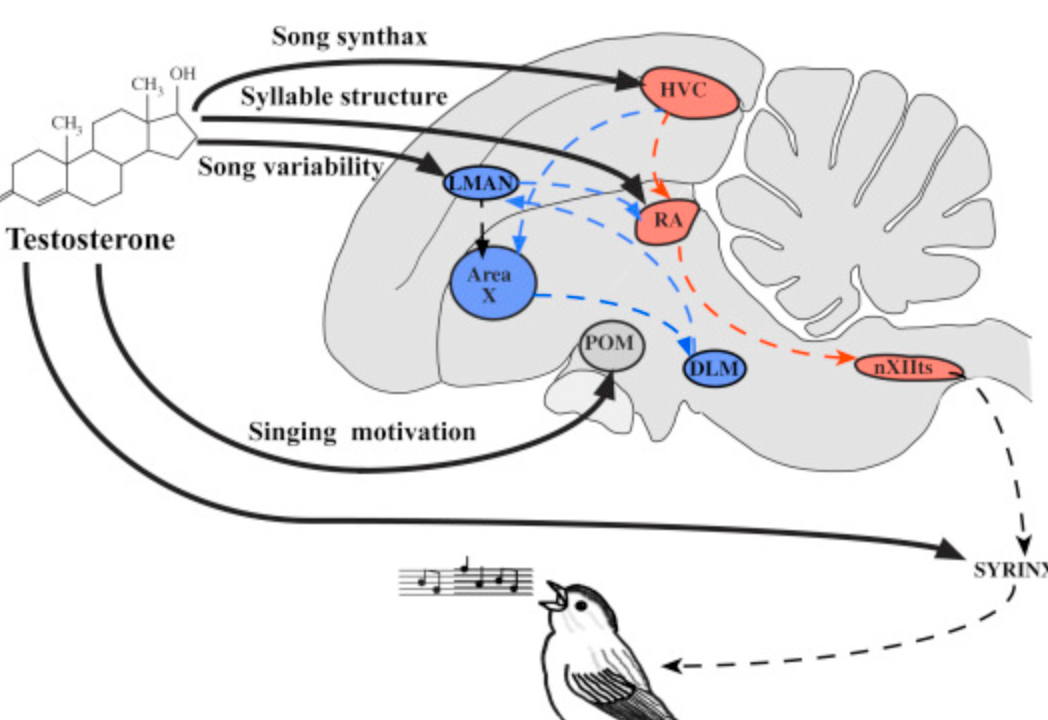
Describe the neural pathway in birds that makes them produce songs.
High vocal centre (HVC) => Robust Archistriatum (RA) => Hypoglossal Nucleus => Syrinx
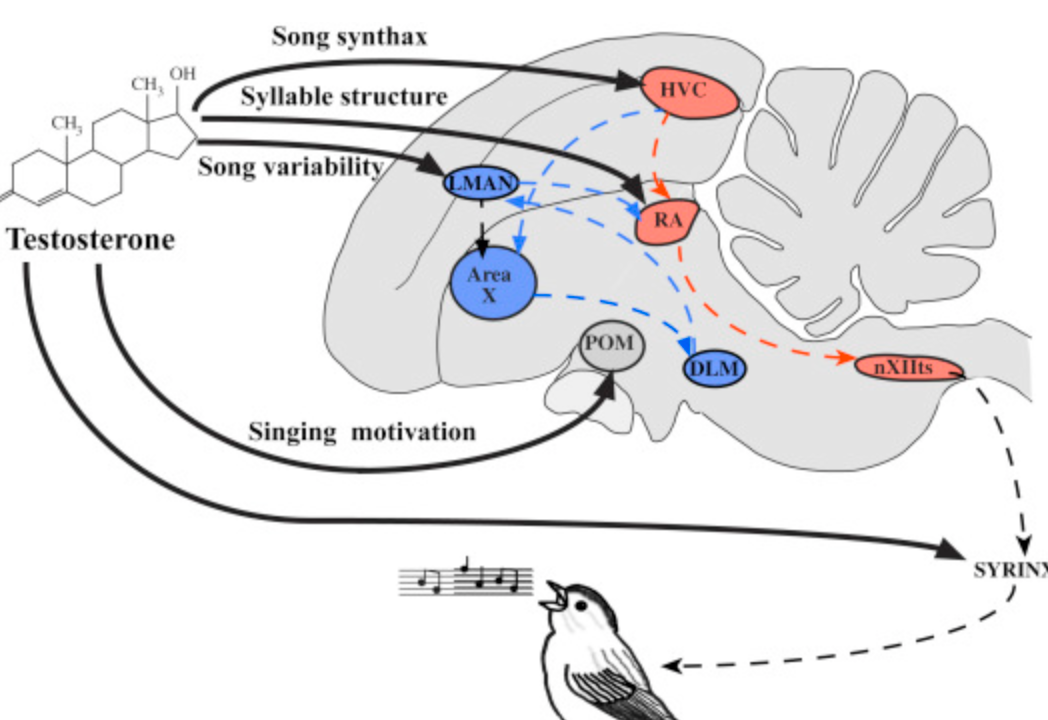
Describe the neural pathway in birds that they use to correct their singing (aka the critic).
High vocal centre (HVC) => Area X => LMAN => Robust Archistriatum (RA)
Describe the sex differences in zebra finch song pathways.
Efferent pathway:
High vocal centre (HVC), Robust archistraitum (RA), hypoglossal nucleus (XIII) are larger in males
HVC has a broader dendrite tree in males
More cells with steroid receptors
Recursive loop:
LMAN larger in males
More LMAN neurons project to RA
Describe the “freaky” hermaphrodite zebra finch in Art Arnold’s lab.
They were studying zebra finches. They had a zebra finch that was half male half female. One side of the body was male, the other female. Also one side had testes, the other ovaries. When they studied the brain they also saw one side was male, one side female (as in their song producing circuits). Since the blood circulation is obviously common in the same bird, this proves that not all sexually dimorphic organisational changes in the brain are due to hormones - chromosomes play a role there, too.

What correction to organisational hypothesis of sexual dimorphism did Art Arnold’s shemale zebra finch provide?
Organisational theory says that hormones shape the male/female brain perinatally. Arthur Arnold always doubted that that’s the only factor.
He had a zebra finch in his lab whose left half was female, right half was male. Left half had ovaries, right half had testes. The bird’s brain was also half male / half female. Since the same hormones were obviously circulating and washing through the left and right side of the brain, this proves that DNA also plays a role in the organisation of the brain.
How frequent are GnRH pulses?
1-2 pulses/hour
List four typical types of mutations affecting reproductive function.
affecting GnRH neuron migration
affecting GnRH synthesis and release
affecting GnRH action (e.g. GNRHR mutations)
affecting gonadotropin synthesis (e.g. LHR, FSHR mutations)
Difference between sexual motivation and sexual performance.
Sexual motivation: interest, drive
Sexual performance: erectile function
They seem to be controlled separately.
How do we measure sexual performance in rats?
mounting (number of times male rat mounts female, or how long before mounts i.e. latency)
intromissions (number, latency)
ejaculations (number, latency)
What happens if your give DHT to castrate rats.
A return of normal erection but not sexual motivation.
Note that T also works, b/c T => (5a-reducatse) => DHT
Which hormone returns normal erection but not sexual motivation in castrated rats. And what receptors are important for that?
DHT. Androgen receptors.
T also works, b/c T => (5a-reducatse) => DHT
Which hormone restores sexual motivation in castrated rats? What receptor does it stimulate?
Testosterone. Stimulates estrogen receptor in the HT.
Lesion to which area in the brain disrupts sexual behaviour in male rats?
Preoptic area
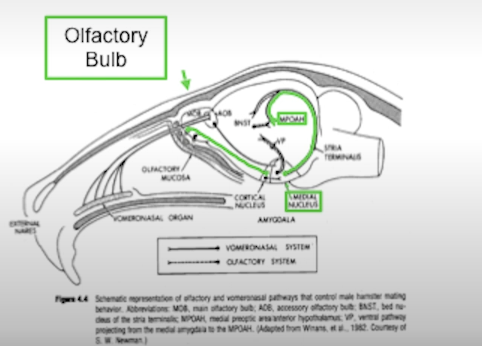
In rats which sense is important for mating beahviour?
Olfaction. Goes into medial amygdala and then relayed to preoptic area (responsible for sexual behaviour in males).
What are immediate early gene?
Immediately early genes are genes that do not require new protein synthesis to be activated. They are activated within minutes. They are typically short-lived. They respond to various triggers e.g. hormones, stress, neuronal activity, even light and sound. Rely on transcription factors already present in cell. They often act as master genes switching other gene son or off. They play a role in neuronal plasticity improving or weakening neuronal connections. They can activate immune cells in response to infection.
Examples: c-fos (growth, differentiation, found in active neurons); c-jun (growth and apoptosis, found in response to stress).
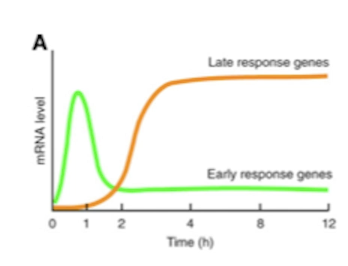
Contrast normal genes and immediate early genes.
Immediate early genes are essentially the first genes that are expressed upon certain signals e.g. hormone. Produced within minutes. They then produce transcription factors necessary for the production of normal genes. Immediate early genes essentially set the stage for the expression of normal genes - but they do not dictate which normal genes are expressed, instead that is decided by additional transcription factors, co-regulators and post-translational modification.
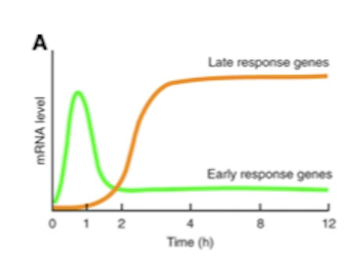
What is fos-mapping?
A widely used neuroscience technique that maps neuronal activation in the brain. Active neurons express immediate early genes first (within minutes). One of those genes is c-fos that is found in active neurons.
How do we study neurons that are activated during sexual behaviour in a rat?
allow e.g. male rat to mate
slice the brain
map for c-fos genes (using e.g. mRNA FISH)
This technique is called fos-mapping. One of immediate early genes “c-fos” is expressed in active neurons within minutes of neuronal activity.
What receptors do we find in the areas fos-mapped after a mating behaviour in male rats?
We find estrogen receptors (ERs) and androgen receptors (ARs).
If we have a male rat and lesion olfactory information going to the medial amygdala on one side of the brain, will the mating behaviour still occur? what if we lesion on both sides? what if we lesion on one side, and block androgen receptors on the other side?
lesion med amygdala on one side - mating occurs
lesion med amygdala on both sides - no mating
lesion med amygdala on one side, but block AR / ER on the other side - no mating (turns out you need those receptors for the mating behaviour - i.e. combination of steroid hormones and olfactory information)
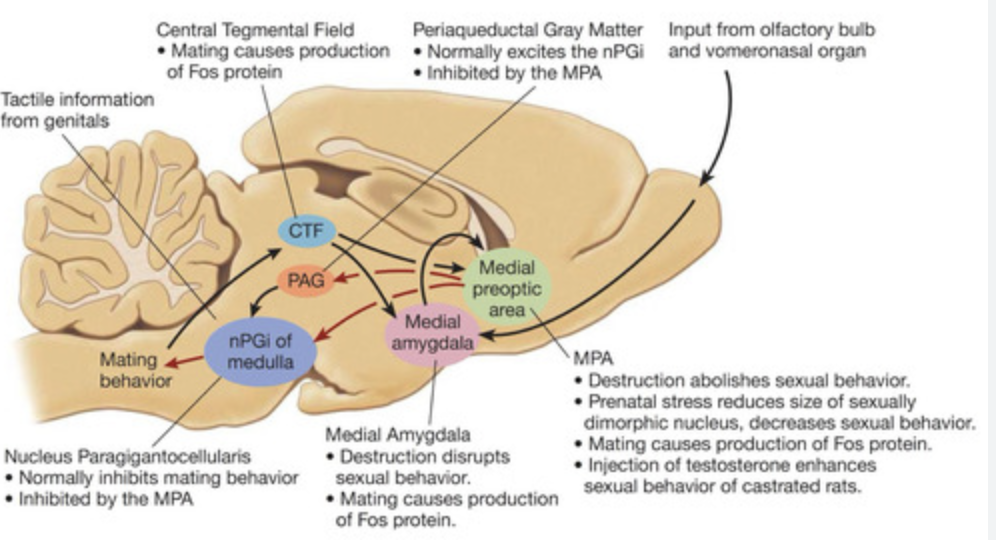
Describe the action of dopamine on male rat mating behaviour. What region?
Dopamine is important for enabling mating behaviour. In preoptic area. POA projects to ventral tegmental area (which is part of the reward pathway).
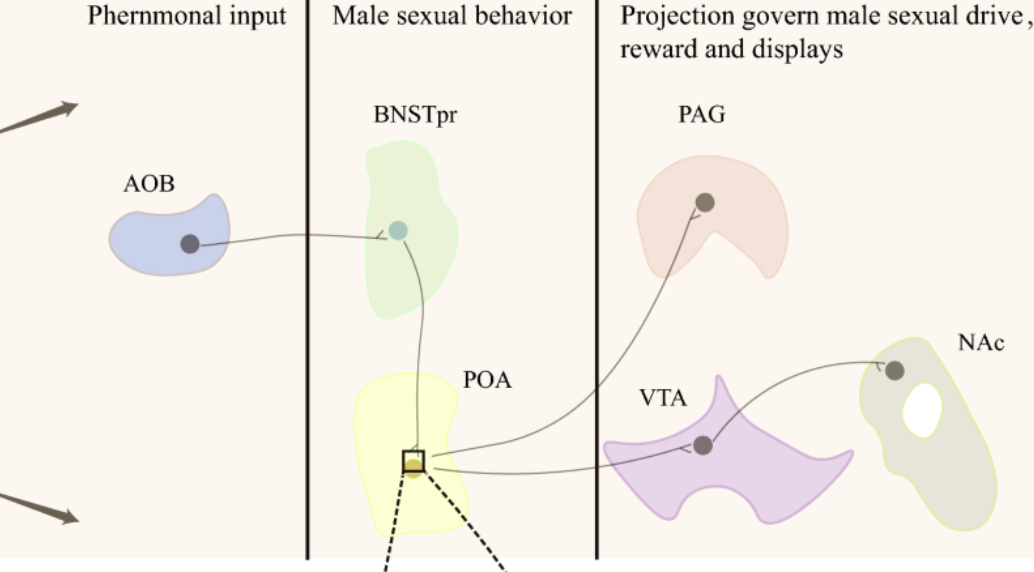
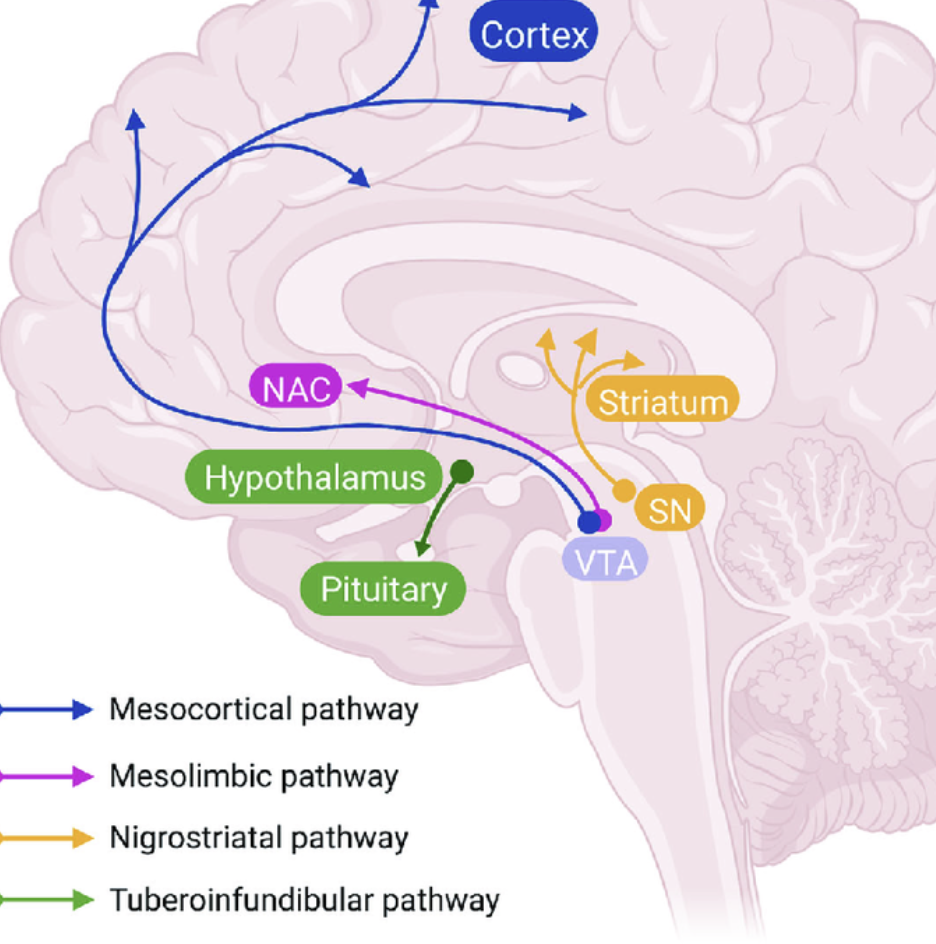
What is the mesolimbic pathway?
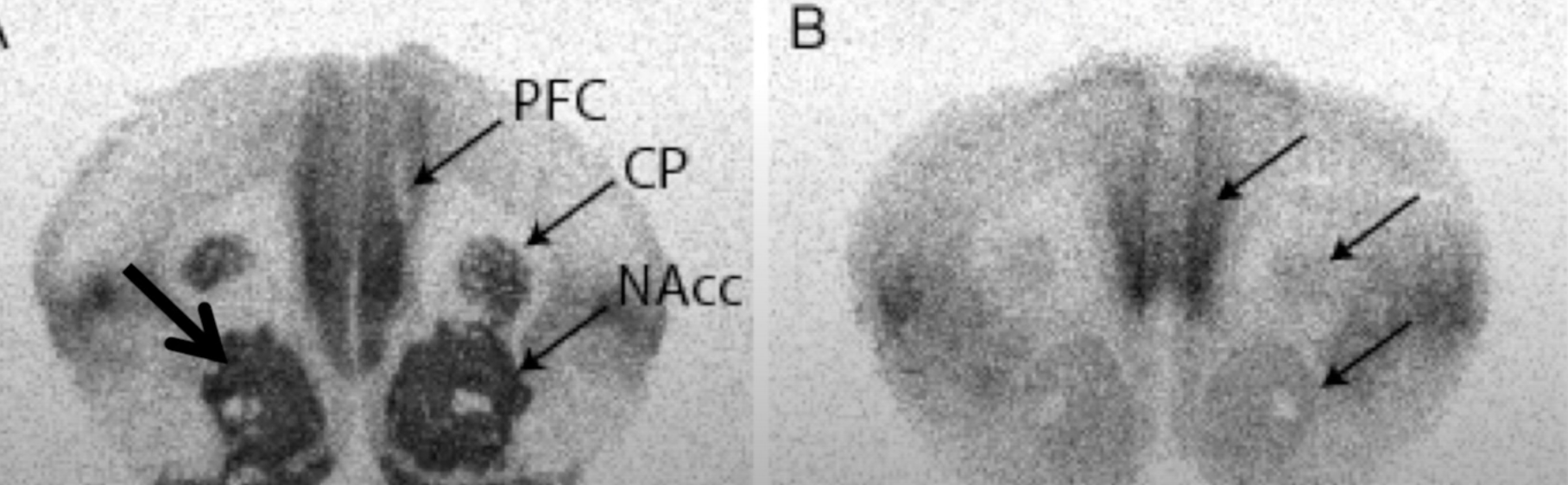
It’s a dopaminergic reward pathway from ventral tegmental area (VTA) to nucleus accumbens (NAcc).
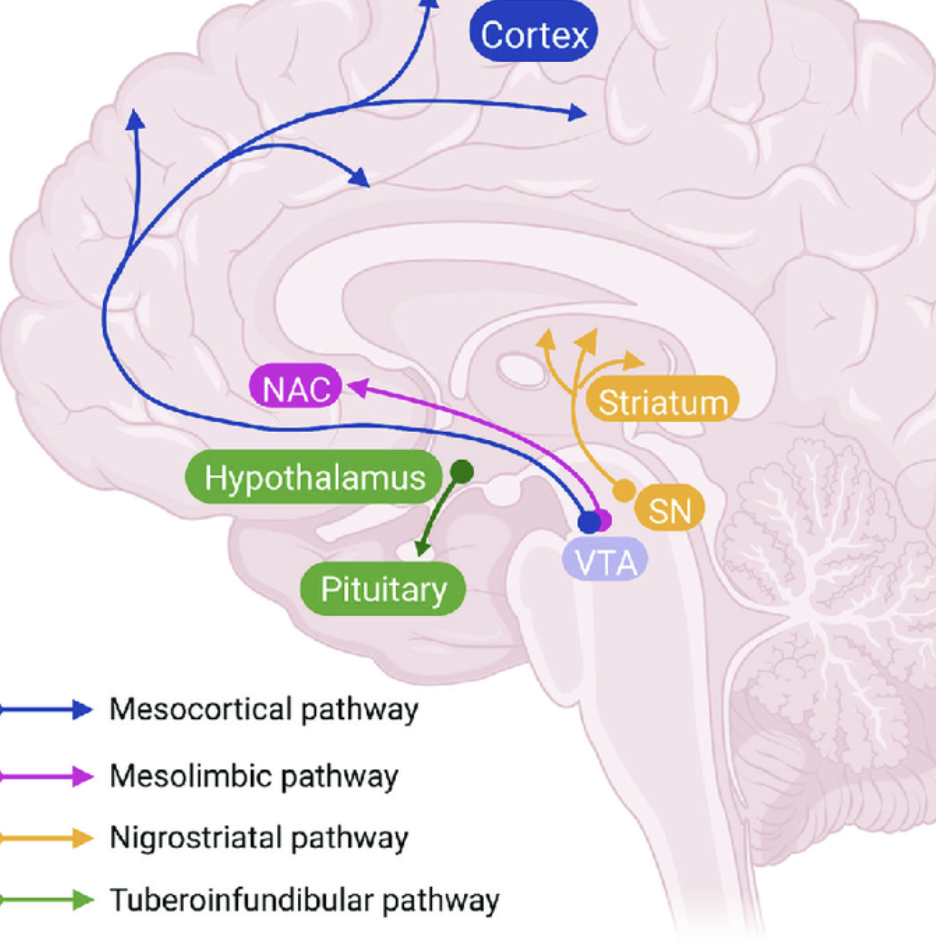
What is the role of testosterone in mesolimbic pathway?
Testosterone enables dopamine action in the mesolimbic pathway. Without testosterone, mesolimbic pathway is poorly activated. It is important because for example in male rats, without testosterone activation, sexual behaviour would not occur as they simply would not feel the “reward” signals.
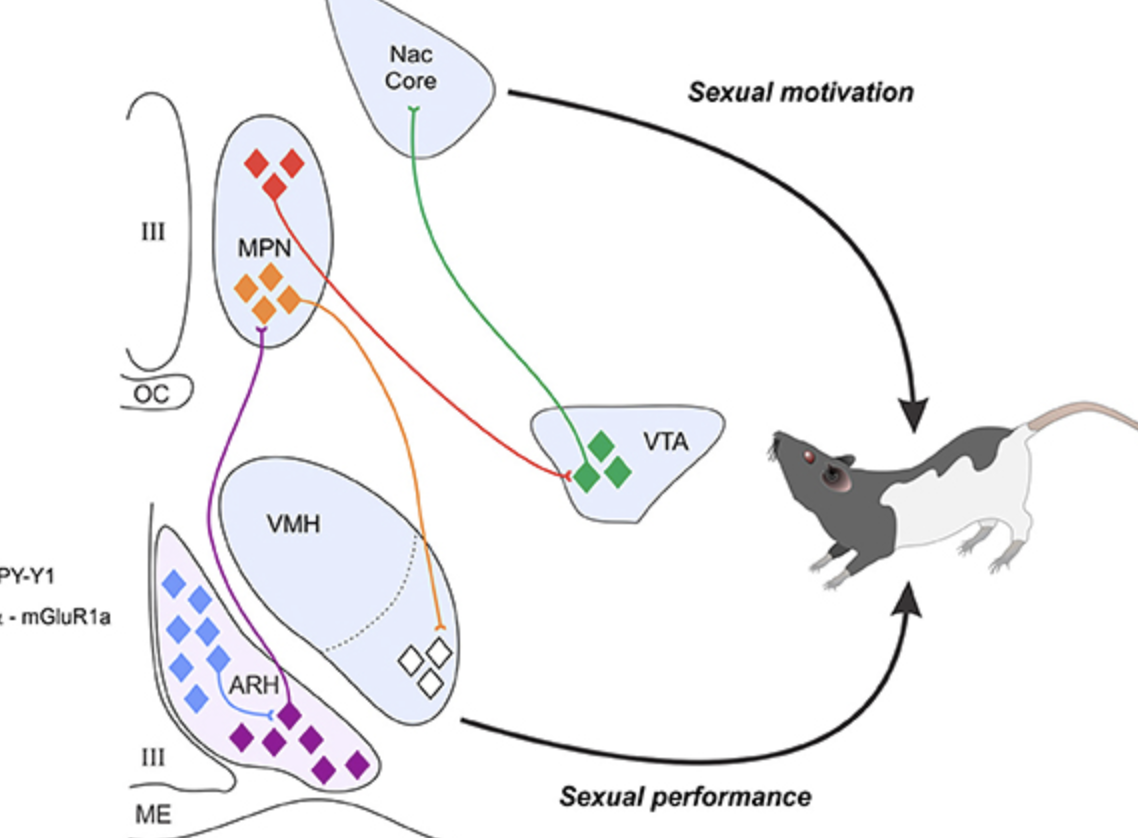
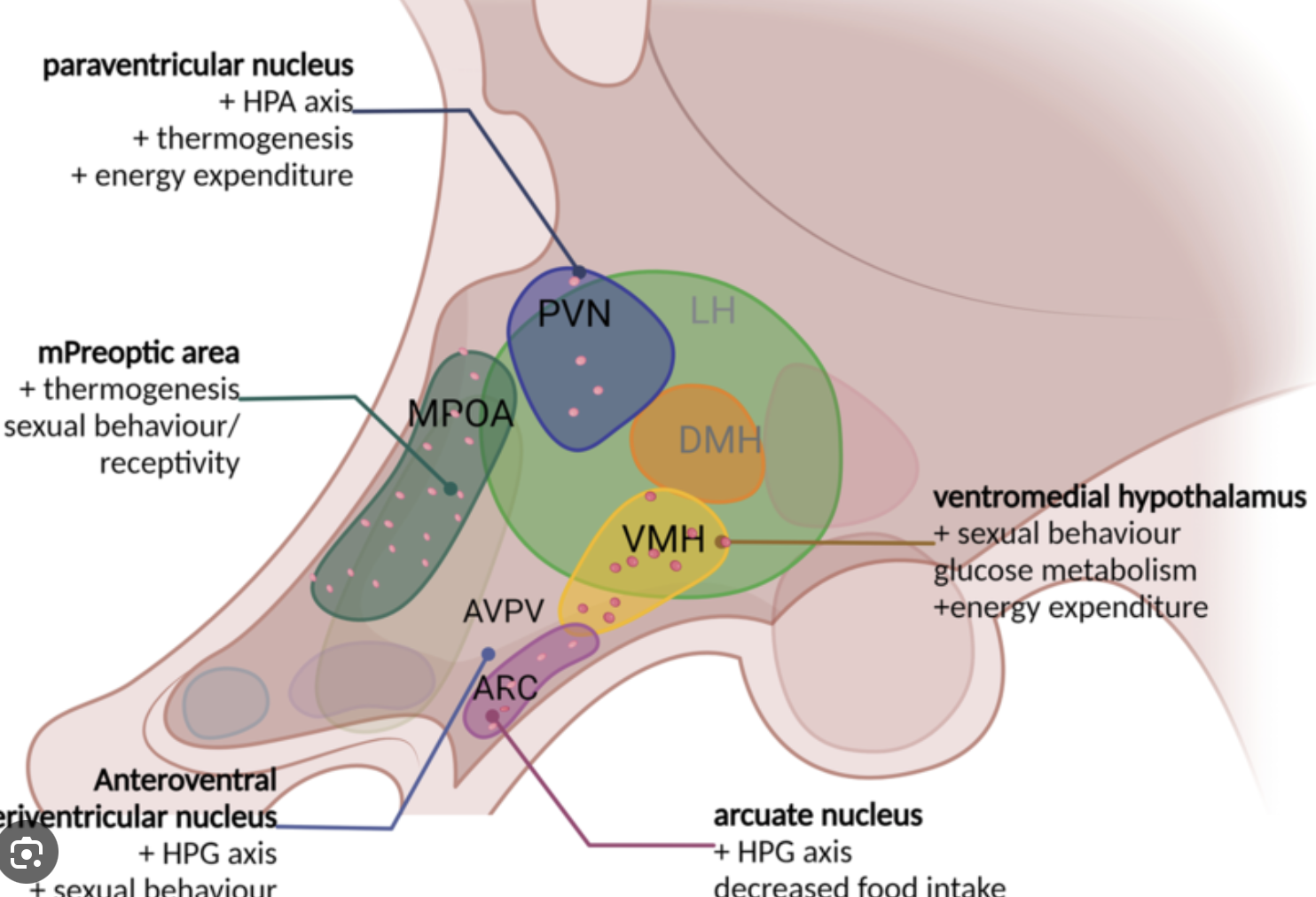
What is VMH? what is its role in sexual behaviour?
VMH is a ventromedial hypothalamus. It’s critical for female sexual behaviour.
VMH is primed by estrogen for lordosis whereas progesterone stimulates sexual behaviour.
VMH is stimulated via mu-opioid receptors (MOR) by the signals from the medial preoptic nucleus that is located in the MPOA.
What is the role of beta-endorphin project from the arcuate nucleus to POA in rats?
Arcuate nucleus is in HT is responsible for metabolism, appetite and reproduction. It has two kinds of neurons:
- POMC neurons (respond to high energy state)
- AgRP/NPY neurons (respond to low energy state)
POMC neurons:
- when energy is high they promote GnRH secretion (signal the body that it’s okay to reproduce)
- when there’s stress / pain they secrete beta-endorphins that (a) reduce pain and (b) signal to POA to reduce GnRH secretion and also to reduce lordosis.
Why do heroin addicts lose sex drive?
Sex drive is in part driven by beta-endorphin projections from arcuate nucleus into the preoptic area (POA). It drives the female sexual receptivity but it inhibits male sexual behaviour.
Also mu-opioid receptors (MORs) become downregulated in heroin addicts.
Why are gonadotropins are lowered in heroin addicts?
Beta-endorphins produced in the arcuate nucleus of the HT regulate GnRH neurons. Heroin mimics opioids and therefore in heroin addicts GnRH neurons are significantly inhibited diminishing sexual drive.
List possible causes of infertility in men?
low androgen production (easy - just hormone replacement)
low sperm production
abnormal development of testes
trauma to testes
drugs could’ve affected testes
infection could’ve permanently damaged testes
autoimmune
Fertility control issues in men?
you’d think giving TRT would inhibit GnRH / FSH but it’s not effective enough
antiandrogens (not effective enough)
cancer drugs (blocks cell division) - but of course no one would take them
barrier (works)
vasectomy (works)
Two pathways activating erection.
local reflex: tactile stimulation, sacral segments of the spinal cord, okay after higher transection
CNS reflex: psychogenic/visual stimulation, thoracic and lumbar segments, spared in case of sacral damage
Does castration lead to complete loss of male sexual behaviour?
It diminishes it but no complete loss. E.g. even children can have erection and they have very low testosterone.
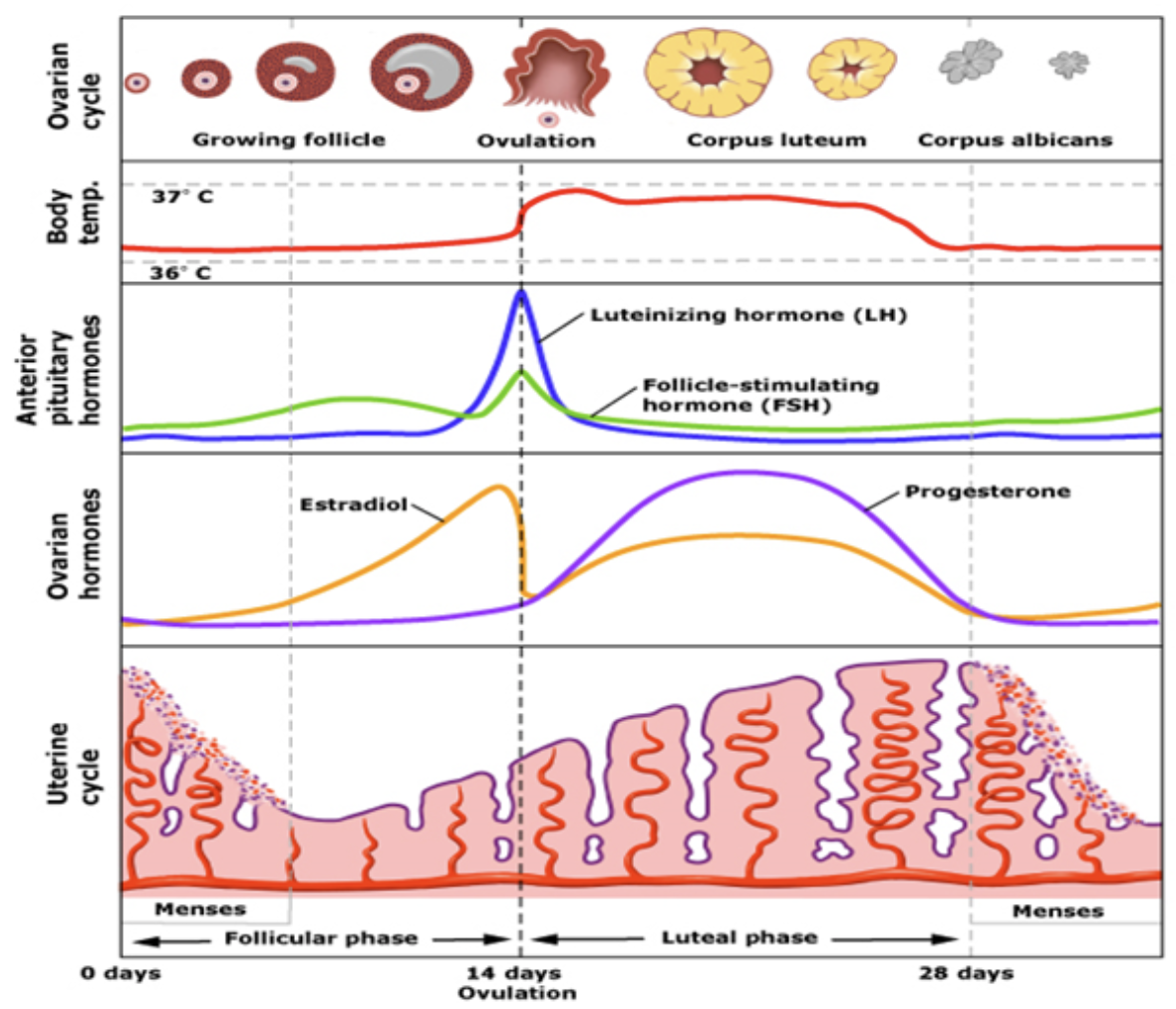
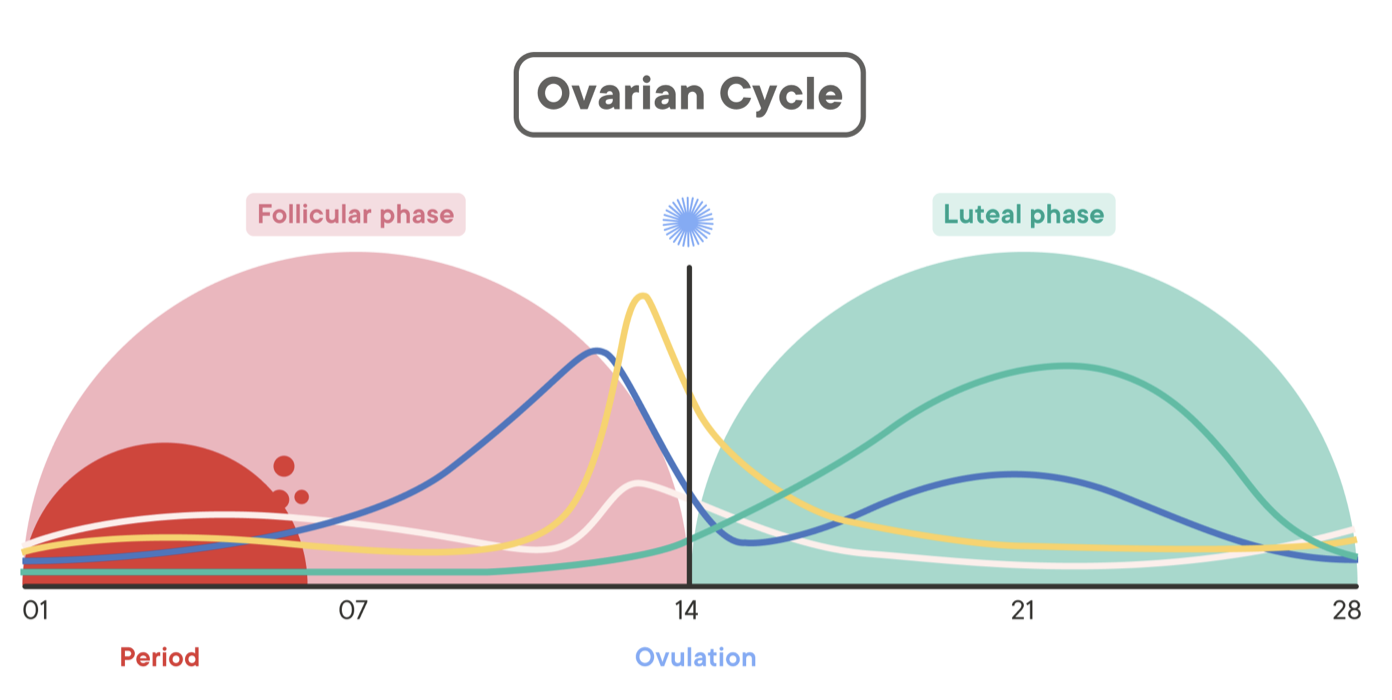
Draw female cycle, note the events, show hormone levels.
FSH initiates development of follicles
Follicles develop and produce increasingly more E2
E2 thickens uterine lining
E2 acts on kisspeptin in AVPV and creates a positive feedback loop on GnRH
GnRH causes a surge of LH
LH causes one follicle to rupture
With that E2 drops
Egg goes into the fallopian tubes
Corpus luteum (what’s left of the follicle) remains
Corpus produces progesterone to maintain uterine lining
If an egg is fertilised it implants into lining, placenta forms which starts producing progesterone and also hCG to keep the corpus alive (and preventing further follicles from maturing)
If the egg is not fertilised, corpus dissolves, progesterone drops, lining starts shedding and the cycle starts again
Explain the dimorphic nature of AVPV.
Anterioventral periventricular nucleus of the hypothalamus (AVPV) has a population of kisspeptin neurons that stimulate GnRH cells
AVPV is part of the positive feedback loop of the female cycle
AVPV is larger in females than males
AVPV in males provides direct and possibly indirect negative feedback from the bed nucleus of the stria terminalis (BNST) in the basal forebrain
BNST regulates stress, anxiety, emotional responses
BNST is larger in males
Explain how exactly the positive GnRH feedback works.
Increasing estrogen from follicles acts on AVPV
In AVPV kisspeptin neurons are activated
Kisspeptin neurons activate GnRH in POA
GnRH produces LH
LH stimulates more estrogen
What is a luteal phase?
Occurs after follicle releases an egg (blastocyst). Humans have spontaneous luteal phase. Rats, for example, don’t.
What is pseudopregnancy?
Actually exists. Elevated progesterone levels but in the absence of an actual blastocyst.
In rats mating cues (even if she mates with a sterile male) would lead to sustained luteal phase.
What can affect the female cycles in animals.
pheromones (female sync cycles)
low energy (stress, low nutrients) shut off pulse generator
seasonal in some species (e.g. deer give birth in spring) so photoperiod affects that too
Explain attractivity, receptivity and proceptivity in rats.
attractivity: signal to specific males (being attractive)
receptivity: lordosis, willingness to be mounted
proceptivity: soliciting males (hopping and darting, wiggling ears)
Can female rat affect the length of intromissions? How?
Yes females do control. After ejaculations they hide from the male for about 7 minutes and then repeat. This is timed with the spikes in progesterone. It ensures more offsprings.
Do hormones affect female mating behaviour?
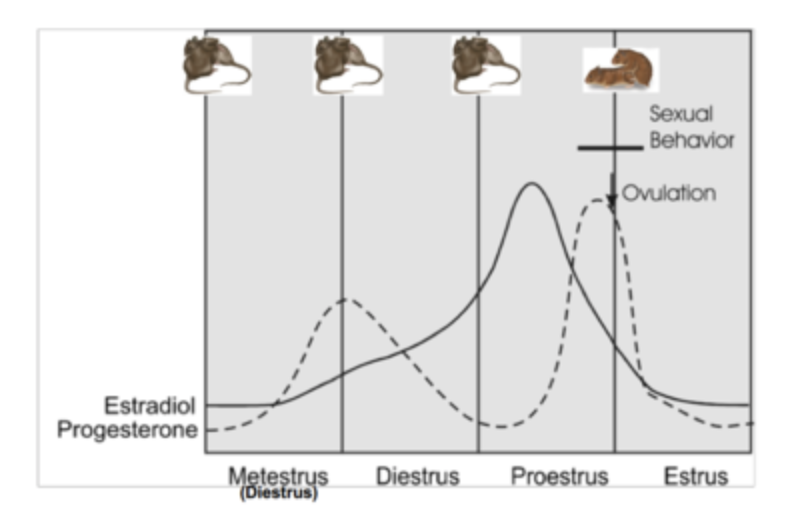
RATS: receptivity coincides with high progesterone release on day 3 of cycle.
HUMANS: high E, T in females increase libido, high P tanks it.
How is preoptic area different in males in terms of connections? Which molecule is involved?
POA has more dendritic spines in males. It seems like prostaglandins (PGE2) are involved.
So it’s actually not testosterone and not even estradiol that masculinises POA, but its’ conversion of:
Testosterone (T) → Aromatase → Estradiol (E₂) → COX2 → Prostaglandin E₂ (PGE₂)
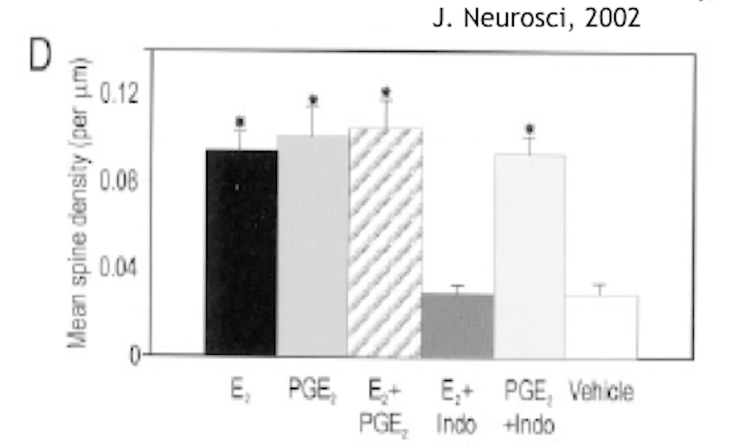
How do COX2 inhibitors affect male and female behaviour?
Preoptic area (POA) is larger in males than females. It was first thought that testosterone affects that, later found that E2 masculinises that area, and even later it was found that it’s prostaglandins that do it:
Testosterone (T) → Aromatase → Estradiol (E₂) → COX2 → Prostaglandin E₂ (PGE₂)
So COX2 inhbitors would prevent male behaviour. it does not affect female behaviour.
Are male and female circuitry in the brain mutually exclusive?
Male and female circuitry in the brain are NOT mutually exclusive. For example T/E2/PGE2 masculinise POA. Their absence allows expression of “female circuitry” genes.
Technically some circumstances may lead to the brain retaining both male and female characteristics.
Where could we inject estrogen to increase receptive behaviour in rats?
In VMH (ventromedial hypothalamus). Increases receptiveness / lordosis.
What happens if we lesion VMH area in rats?
Would disrupt receptiveness in sexual behaviour of female rats (i.e. no lordosis).
What’s VMH responsible for? How is it different between males and females?
ventromedial hypothalamus
responsible for metabolism, thermoregulation, aggression, reproductive behaviour
in females VMH is essential for sexual receptivity
in males VMH it drives sexual motivation (but less so than POA) and aggression
VMH in males is larger but mostly due to more connections, # of neurons is the same
VMH in females has more ER receptors
Describe lordosis pathway.
1) E, P prime ventromedial HT (VMH)
2) Male stimuli (touching flanks) stimulate VMH
3) VMH => PAG => MRF (medullary reticular formation) => specific muscles
Note that MRF is important for posture control (among other things).
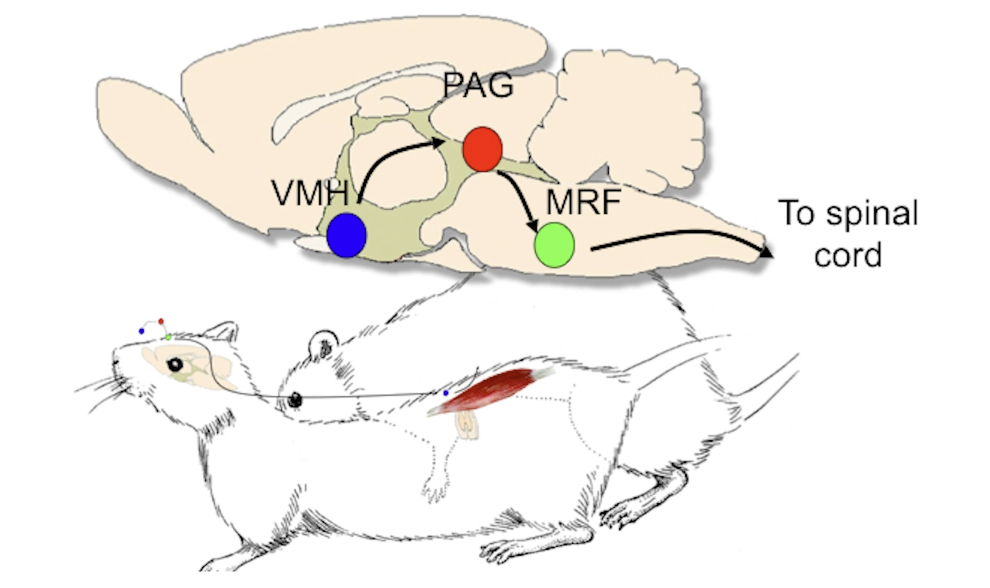
When we inject a pseudorabies virus in lumbar muscle responsible for lordosis, where do we expect to see changes in the brain and why?
We use this virus to trace the pathway all the way from the back muscle to the brain. We ultimately see that the main command centre is in the ventrolateral nucleus of VMH (ventromedial hypothalamus) i.e. VL-VMH, which then controls PAG, then medullary reticular formation (MRF) which is responsible for posture.
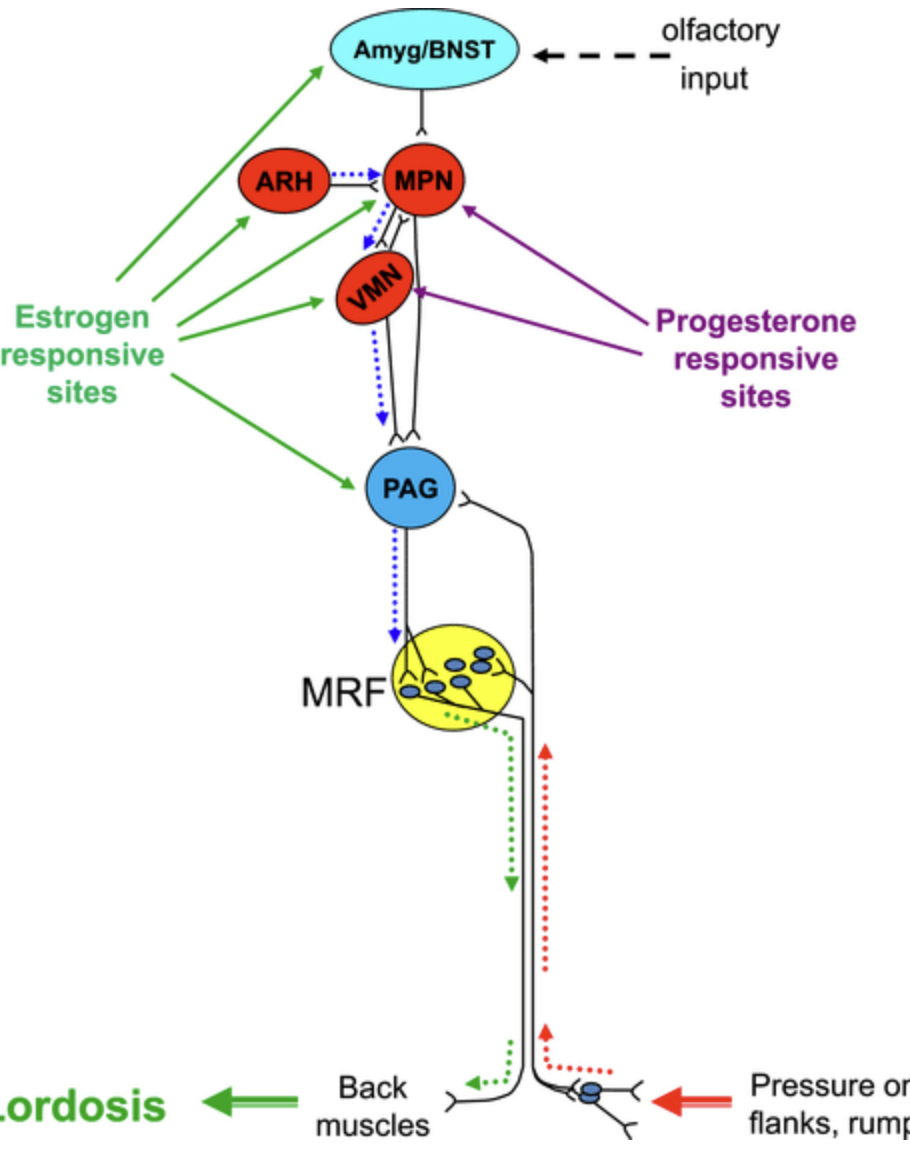
Are the neurons with estrogen receptors in VL-VMH are the same neurons controlling lordosis?
Ventrolateral nucleus of ventromedial hypothalamus (VL-VMH) controls PAG which controls medullary reticular formation (MRF) which is responsible for lordosis posture in female rats.
We found that there’s some overlap of ER and controlling neurons, but mostly they are separate. So neurons with estrogen receptors probably talk to neurons that then control PAG => MRF => back muscles.

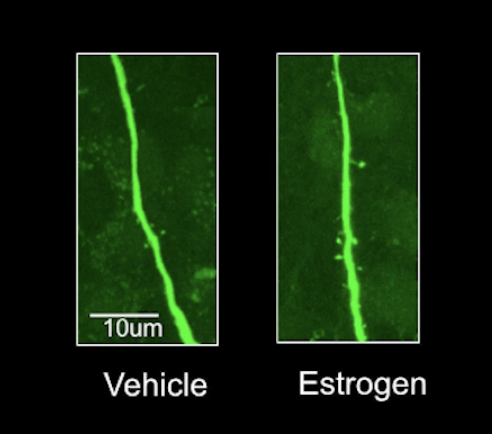
How does estrogen treatment change VMH?
Treating ventromedial nucleus of the hypothalamus induces synaptic terminals and increases dendritic spine density in vlVMH (ventrolateral VMH) but not in dorsal portion of VMH.
Note that these neurons that grew dendritic spines did NOT contain estrogen receptors!!! VERY IMPORTANT. Most likely estrogen sensing neurons transsynaptically induced other neurons to grow spines.
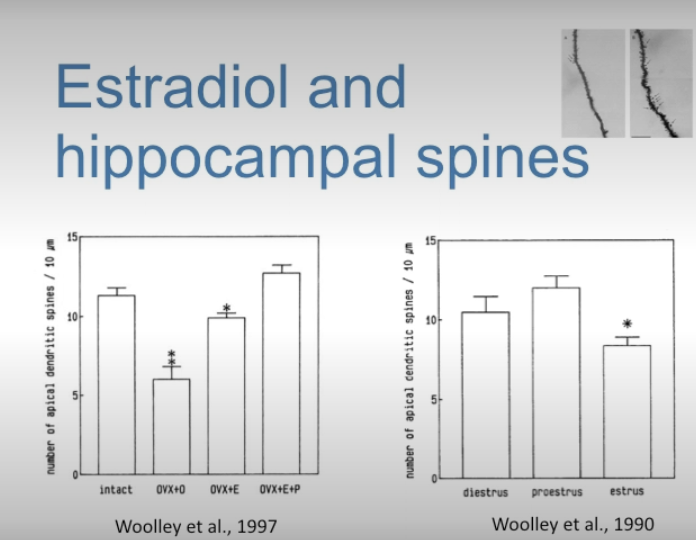
How do estrogen and progesterone act on apical dendrites in the hippocampus?
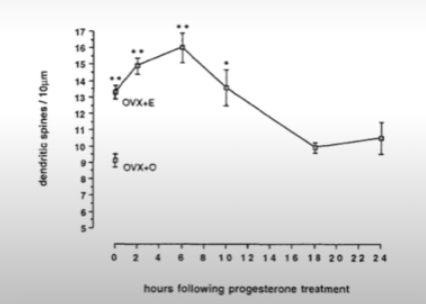
Work of Woolley (in Brisbane lab). She found that both estrogen and progesterone increase dendritic spines in apical dendrites of the hippocampus.
Is increase of dendritic spines in apical dendrites of the hippocampus mediated by estrogen receptors or another kind of receptors, and how was this proved?
Estrogen receptors. Proved by blocking ER with estrogen receptor antagonist - dendritic spines did not form.
How does progesterone affects dendritic spines on hippocampal neurons?
Estrogen increases dendritic spines. Giving progesterone on top of estrogen increases dendritic spine density even further but then after a few hours it actually reversed that increase below even the starting point. This is called biphasic effect.
For example giivng RU486 (that blocks progesterone receptors) actually brings the level of dendritic spines back up (during rat’s estrus).
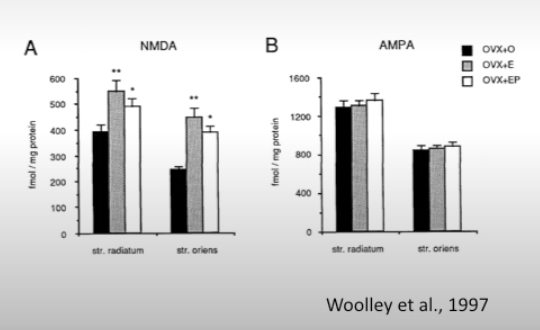
How does E, P hormone treatment affect glutamate receptors in the hippocampus.
No effect on AMPA receptors. But increases NMDA receptors. In postmenopausal women E treatment will improve cognitive abilities. Note that hippocampus forms long term memory, is crucial for learning, connects memory and emotions (by communicating with amygdala, important for spatial navigation)
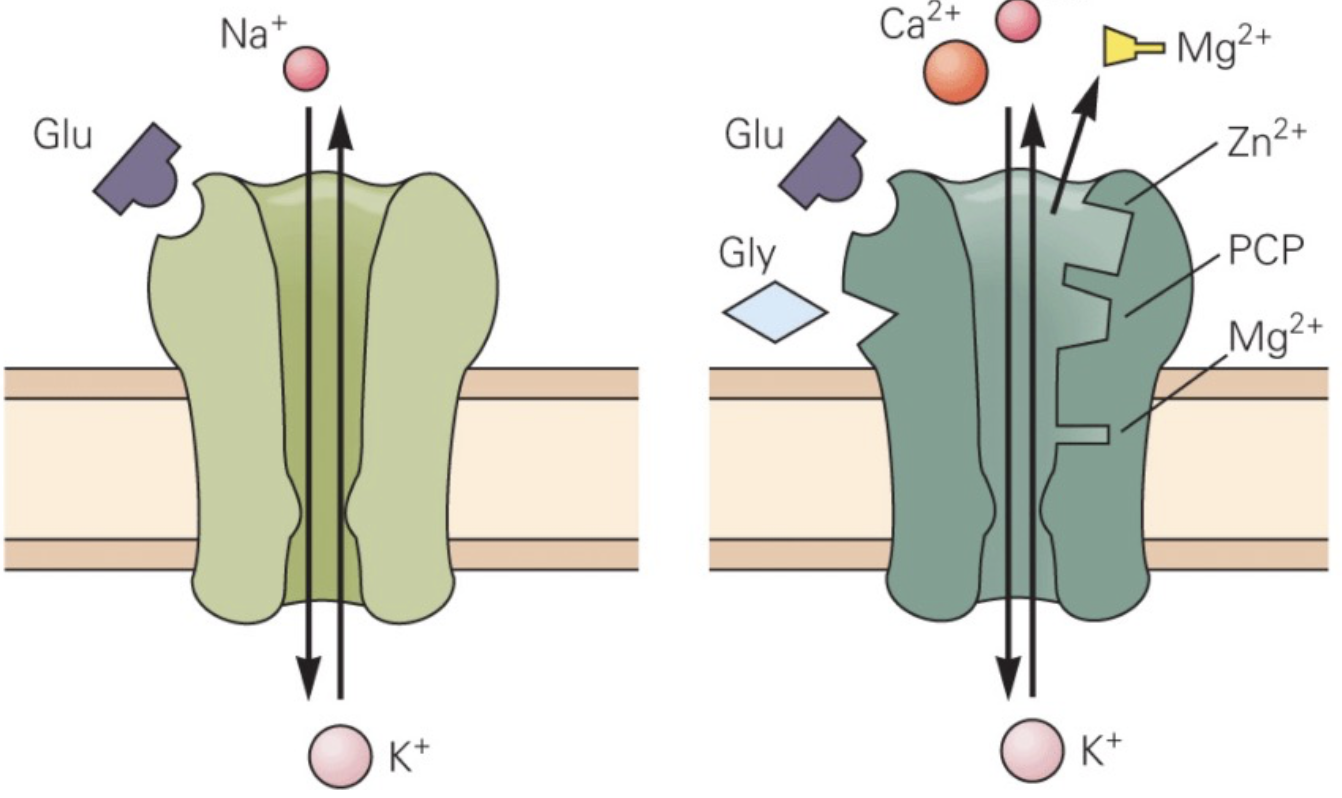
What are AMPA and NMDA receptors?
Both are glutamate receptors.
AMPA are fast receptors. Upon binding glutamate they allow Na+ in causing depolarisation.
NMDA are slower receptors. They act as coincidence detectors (and thus important for associative learning) - they require that glutamate binds to the receptor and that Mg++ is ejected from the receptor by the post-synaptic depolarisation. They allow Na+ and Ca++ in. Ca++ is central to long-term changes initiated by this receptor (synaptic plasticity).
How does E and P treatment of hippocampus change the number of excitatory inputs to hippocampal neurons?
It not only increases the number of dendritic spines, but also the number of inputs. So that some spines have two or more inputs!
Which animals / species are known to provide parental care?
Mammals and birds.
Three types of parental strategies.
nesting strategy (altricial young) .. birds
leading and following (precocious young) .. e.g. deer
clinging and carrying (semi-altricial young) .. e.g. primates
Altricial = born helpless, can’t see or hear, can’t move, can’t feed themselves, possibly can’t regulate temperature
Semi-altricial = can see and hear, can’t move
Roles of hormones during pregnancy.
prepare for milk production
adjust nervous system to prepare to exhibit parental behaviour
during pregnancy avoid expelling fetus
closer to end of pregnancy prepare for expelling fetus
Do females (birds, etc) show interest in taking care of young, in nesting, etc.
Yes, but only those who are pregnant or have babies - this is due to hormones. Those that’ve never been pregnant are typically not interested.
In people - pregannt women often become interested in picking nursery, etc.
Are birds biparental?
90% are. But some have only mother looking after babies (e.g. ducks) or only father (e.g. jacanas). And some are parasites (like cuckoo birds and corbirds).
What is broodiness?
Nesting behaviour, sitting on eggs, protecting, warming, covering with wings.
Which hormone activates broodiness?
Prolactin. Broodiness = nesting behaviour, sitting on eggs, protecting, warming, covering with wings.
Note cowbirds (parasites) are insensitive to prolactin.
Do hormones influence behaviour or behaviour influences hormones?
It goes both ways! It creates self-propagating cascade.
Which area is important for parental behaviour in birds?
Preoptic area. It contains prolactin receptors.
Which hormone in birds is responsible for producing crop milk?
Prolactin
Which hormone in birds is responsible for parental behaviour?
Prolactin